
Extracorporeal membrane oxygenation for severe pediatric respiratory failure
Introduction
In children with severe acute respiratory distress syndrome (ARDS), extracorporeal membrane oxygenation (ECMO) is most often used as a rescue therapy. ECMO uses an extracorporeal circuit with a pump and an oxygenator to support life in cases of refractory cardiac and/or respiratory failure. Blood and gas flow in counter-current directions within the oxygenator, and gas exchange occurs by diffusion across the membrane. Blood flow and fraction of inspired oxygen in the circuit determine arterial oxygenation. The sweep gas flow through the membrane removes carbon dioxide (CO2). In the absence of significant hemodynamic instability, venovenous (VV) ECMO is generally sufficient to augment gas exchange allowing ventilator settings to be weaned and, thus, potentially reducing ventilator-induced lung injury and oxygen toxicity. However, as blood is returned to the central venous system, VV ECMO does not provide direct circulatory support. In children with concomitant hemodynamic instability or when there is inadequate oxygenation with VV ECMO, venoarterial (VA) ECMO can be employed. It must be emphasized that ECMO itself does not heal or treat the underlying condition(s), but it provides cardiac and/or respiratory support to allow the pathophysiology to resolve without toxic ventilator and/or vasoactive agent/inotrope support.
Although ECMO is generally used as a last resort for those with high mortality, survival over the last 10 years for respiratory failure requiring ECMO support is approximately 53–62% for children and 63–70% for neonates (1). Experience with the H1N1 influenza pandemic suggests that ECMO may be an important management strategy in future viral epidemics/pandemics with reported survival rates of greater than 70% (2,3). In this review, we describe the historical perspective of respiratory ECMO and examine the current evidence and experience with regard to respiratory ECMO and clinical outcomes and consider future challenges the critical care/ECMO team needs to overcome to further refine the application of this technology in the most critically ill of infants and children.
Methodology
This narrative review presents the history, development, and current advancements in the field of pediatric respiratory ECMO, in particular VV ECMO. The aim of this review is to provide a critical update on the advancements in pediatric respiratory ECMO. Studies with subjects within the pediatric and neonatal age group (<18 years) supported on ECMO for the indication of respiratory failure are included. Studies that include both cardiac and respiratory indications are also included as long as data were reported for the respiratory group. The use of ventricular assist devices, Berlin heart devices, and extracorporeal CO2 removal was not evaluated.
In view of the limited medical literature, we include all published studies, prospective as well as retrospective, to comprehensively assess this topic. We conducted the search on PubMed (MEDLINE) using MESH terms including “acute respiratory insufficiency”, “ARDS”, “ECMO”, “paediatrics”, and “neonate” from 1998 to August 2016. No language restriction was used.
Titles and abstracts were screened for relevance. Subsequently, full text articles of the relevant abstracts were retrieved and appraised. Information was, thus, gathered, synthesized, and summarised in this review focusing on the following subtopics: indications and contraindications, short-term outcomes, long-term outcomes, and novel management approaches. In this manuscript, short-term outcomes are defined as outcomes within the PICU stay including complications related to ECMO cannulation and maintenance, bleeding, ventilation indices and PICU mortality. Long-term outcomes are defined as outcomes beyond the PICU stay including 1-year survival, neurologic and cognitive sequelae, functional and quality of life.
Results
Historical perspective
The first successful report of ECMO in severe respiratory failure was described more than 40 years ago (4). In this report, ECMO was used for a total of 75 hours in a 24-year old man with ARDS secondary to major trauma. During this initial use of ECMO, the fraction of inspired oxygen (FiO2) on the conventional mechanical ventilation (CMV) was reduced from 1.0 to 0.60, and the peak inspiratory pressure was reduced from 60 to 35 cmH2O. Subsequently, a randomized controlled trial of CMV vs. ECMO was performed in 90 adult patients with severe hypoxemic respiratory failure and showed no survival benefit [4/42 (9.5%) vs. 4/48 (8.3%)] (5). During this period, interest in adult ECMO waned, but reports of successful ECMO use in neonates started to emerge. The first report of the use of ECMO in neonates appeared in 1977 describing 16 moribund newborn infants with respiratory failure who were supported on ECMO (6).
In a subsequent randomized study of 39 neonates with severe persistent pulmonary hypertension and respiratory failure, infants supported with ECMO had an overwhelming higher survival rate as compared to those in a conventional treatment group [28/29 (97%) vs. 6/10 (60%), P<0.05] (7). The UK Collaborative ECMO Trial Group then conducted the largest prospective study of ECMO in 185 neonates with severe respiratory failure (8). This study demonstrated that 30/93 (32.2%) and 54/92 (58.7%) infants in the ECMO and conventional treatment arms died, respectively. This translates to a relative risk of 0.55 [95% confidence interval (CI), 0.39–0.77, P=0.0005] and a number needed to treat of 3–4 infants to achieve one extra survivor. Following the results of these studies, neonatal ECMO for respiratory failure became commonplace. The neonatal respiratory population has formed the largest group of patients supported in the data reported to the Extracorporeal Life Support Organization (ELSO) since 1987 (1).
The experience obtained from neonates with severe respiratory failure slowly encouraged the use of this support modality in the pediatric population (9,10). The number of pediatric respiratory ECMO runs increased from the late 1980s to the early 2000s and remained fairly stable until the upturn in ECMO use related to the 2009 H1N1 pandemic (11,12). The level of evidence with regard to ECMO in children with severe respiratory failure remains low with the majority of the contemporary published literature relying on registry and large single center data and experience. A review focused on timing of initiation and decannulation of ECMO, and ancillary therapies (e.g., bronchoscopy, nutrition, fluid management) highlighted the limitations of contemporary medical evidence with regard to the use of ECMO in pediatric respiratory failure (13).
Expanding indications and shrinking contraindications for respiratory ECMO
Traditionally, ECMO was employed in the neonatal population for conditions such as meconium aspiration syndrome, congenital diaphragmatic hernia, persistent pulmonary hypertension of the newborn (PPHN), and in the pediatric population for conditions such as severe pneumonia and ARDS. While these pulmonary parenchymal diseases continue to be common indications, there are increasing reports of ECMO use for other clinical situations such as upper and lower airway obstruction and pulmonary, foreign body aspiration, and hyperleukocytosis syndrome.
Mediastinal masses invading or compressing any part of the major airways may make intubation and tracheostomy potentially dangerous and in certain circumstances, not helpful. ECMO support can be deployed until chemotherapy or steroids are administered to shrink the tumour (14). In severe status asthmaticus, widespread and refractory bronchoconstriction results in ventilation-perfusion mismatch and hypercapnic respiratory failure. A query of the ELSO registry identified 64 children treated with ECMO for status asthmaticus. Their median partial pressure of CO2 before cannulation was 130 (range, 102–186) mmHg (15). Mortality in this group was low at 6%. The literature also reported of patients in whom ECMO was used in aspiration of substances such as sand and baby powder (16,17). In these cases, ECMO provided a means for gas exchange while the foreign substance was removed by bronchoscopic toileting and allowed the airway inflammation to settle.
Diseases affecting the pulmonary circulation have also been treated with the use of ECMO. In severe pertussis, hyperleukocytosis causes a hyperviscosity syndrome and obstruction of pulmonary arterioles. Aggregation of leukocytes and thrombosis within the pulmonary circulation causes refractory pulmonary hypertension and eventual right heart failure. Retrospective studies reported high mortality rates in patients with severe pertussis treated with ECMO (58–70%) (18). In the same way, adhesion, aggregation and thrombosis of sickle erythrocytes in the pulmonary microcirculation of patients with sickle cell disease cause respiratory failure amenable to rescue by ECMO. A query of the ELSO registry identified 45 pediatric patients with sickle cell disease rescued with ECMO for respiratory failure between 1996 to 2012. Mortality in this group was 36%. In line with the increasing collective experience in the utilization of ECMO in respiratory conditions that are considered “non-parenchymal” disease processes, indications for respiratory ECMO are most likely to increase further over time
Life limiting co-morbidities were once considered to be contraindications to ECMO. In the early years of ECMO, the presence of major co-morbidities, such as oncologic diagnoses, solid organ transplantation, hematopoietic stem cell transplantation, and primary immunodeficiency states, were considered as contraindications to ECMO support. This “dogma” has slowly changed as a review of the ELSO registry revealed the proportion of children with co-morbidities in whom ECMO support was offered increased from 19% in 1993 to 47% in 2007 (19,20). Despite the presumably higher risk population in the later cohort, the mortality rate remained unchanged. The two most common co-morbidities of children supported on ECMO in this report were acute kidney injury (10%) and chronic lung disease (9%).
This trend is substantiated by the result of a study involving 131 ECMO centres surveying whether oncologic diagnoses should be considered a contraindication to ECMO. Of the 118 centres that responded, 92 (78%) stated that malignancy should not be a contraindication to respiratory or cardiac ECMO (21). Although ECMO is increasingly offered to children with significant co-morbidities, the intensivists must be aware that the overall outcome of this specific subpopulation of children supported with ECMO is generally worse as compared to those without a significant past medical history. A report from the ELSO registry in 2008 specifically looking at survival outcomes of children with immune compromised conditions (e.g., immune deficiency, malignancies, solid organ and bone marrow transplant), revealed the overall survival was lower in children with an immune compromised state than those with normal immune systems [57/183 (31.3%) vs. 1,550/2,696 (57.5%), P<0.0005] (22). The patients who seem to have the most dismal chance of survival when supported on ECMO are those with hematopoietic stem cell transplantation with published survival rates ranging from 0 to 5% (19,22). An updated analysis from the ELSO registry in 2014 involving on children with hematopoietic stem cell transplantation reported a total of 21 patients supported on respiratory ECMO with a marginally improved overall hospital survival of 10% (23).
Genetic syndromes may also be considered as relative contraindications for ECMO support. However, data extracted from the ELSO registry over a period of 30 years (1983–2013) showed that 323 patients with Trisomy 21 were treated with ECMO (24). This was mainly for PPHN in the neonatal age group and viral pneumonia in the pediatric age group. Survival was comparable with patients without Trisomy 21 (67% vs. 71%, P=0.09) (24). A recent case report even described the use of ECMO as respiratory support for an infant with Pompe’s disease during RSV infection while treatment of the underlying disease with enzyme replacement was co-administered (25). Indeed, there are other scattered case reports describing the experience of the use of ECMO in severe respiratory failure in infants with other genetic conditions (26,27). We expect that the experience of the use of respiratory ECMO in children with underlying genetic syndrome will continue to increase over time.
Short-term outcomes
Over the past decades, there has not been a significant change in overall survival (mortality rates 47–58%) of children supported on respiratory ECMO (19,20,28). Instead of focusing solely on mortality as an outcome, a growing number of studies have investigated the impact of ECMO management on other clinically relevant outcomes such as morbidities in ECMO survivors.
Over the past few years, there is an increasing trend in the use of VV ECMO in children (13). In children with severe respiratory failure without significant cardiac dysfunction, VV ECMO offers potential advantages over VA ECMO. These short-term outcome benefits include a reduced risk of neurological complications, preservation of the carotid artery, and enhanced oxygenation of the pulmonary and coronary vasculature (29,30). Available data suggest that VV ECMO confers an additional absolute survival advantage of 19% as compared to VA ECMO when utilized in children with severe respiratory failure, and VA ECMO was independently associated with increased odds of CNS injury compared to VV ECMO [odds ratio (OR), 1.6 (95% CI, 1.1–2.3)] (19,30).
Besides cannula configuration, the duration of ventilation prior to ECMO support and presence of co-morbidities (as previously described) have also been associated with increased mortality (19). Children who were ventilated for 14 days or less had a survival of 56–61% compared to 38% in children who are ventilated for longer than 14 days prior to cannulation for ECMO (19). This is most likely due to on-going ventilator induced lung injury in addition to the initial lung disease (31). However, as lung protective ventilation strategies are increasingly adopted, this threshold of 14 days may not necessarily hold true in the future (32).
The most recent ELSO review reported short-term morbidities specifically among respiratory ECMO cases (28). The most common were cannula site (17%) and surgical site bleeding (13%), which seem to occur more frequently in children as compared to neonates and adults (28). Intracranial haemorrhage and seizures occur 5–9% of time and are more frequent in neonates (28). Another retrospective ELSO report among patients with respiratory failure identified independent risk factors for central nervous system complications in patients who were younger and those who had a low pH prior to cannulation, pre-existing infection, cancer, renal failure, liver insufficiency, high risk pulmonary diagnosis, or VA configuration (30).
Long-term outcomes
Limited long-term outcome data in children previously supported on ECMO exist, and all available follow-up studies performed in the context of ECMO support are for both cardiac and respiratory indications (33,34). The UK Collaborative ECMO Trial Group provided data on survivors of neonatal ECMO (35). The majority of the patients in this cohort had respiratory indications for ECMO 49/62 (79%). They found that 13/62 (21%) patients were disabled (hearing loss, vision loss, functional loss) and 4/62 (6%) were impaired at 1 year of age (35). The same group of children were followed up to 4 years of age, and 30/62 (48%) were found to be disabled and 18/62 (18%) impaired (36). At this stage, cognitive ability measured by the British Ability Scales II was abnormal in a third of survivors. Hyperactivity and conduct difficulties measured by the Goldman strengths and difficulties questionnaire were identified in another third of survivors. Up to 12% and 13% of survivors had significant sensorineural hearing loss and abnormal vision, respectively. At 7 years of age, 56/62 (90%) of patients remained available for long-term neurocognitive assessment (37). Using the same assessment tools, 24% had cognitive levels below the normal range, 18% had behavioral difficulties, 28% had hearing abnormalities, and 16% had visual abnormalities. Abnormal neuromotor development was present in 55%. These series of longitudinal studies also studied a control group who were managed with maximal conventional management. It is important to point out that even though there were significant long-term morbidities in the ECMO arm, the frequency and severity of morbidity was no different than the control arm.
The Dutch ECMO follow-up team studied a single cohort of neonatal ECMO survivors and compared their developmental performance to population norms (38). Five years old ECMO survivors had normal development in 49%, severe disabilities in 13%, and combined motor and cognitive/behavioral disability in 9% (38). At 8 years of age, the investigators found the mean intelligence quotient score of ECMO survivors (99.9±17.7) was not different than normal children (39). Subsequently, following up to 12 years of age, they found that ECMO survivors had a lower pediatric quality of life (PedsQL) score [mean (standard deviation), −1.26 (1.53), P<0.01] and 22% had motor problems in the form of manual dexterity, ball skills, and balance skills measured by the Movement Assessment Battery for Children (38). The Dutch group also studied neuroimaging, pain sensitivity and neuropsychological functioning in school aged ECMO survivors but found only minor abnormalities including poorer memory in the ECMO arm (40). Unfortunately, specific data on long-term outcomes in children supported on ECMO for respiratory indications are lacking. This highlights the need for additional follow-up studies of children supported on ECMO for severe respiratory failure. A summary of pediatric and adult studies on long-term outcomes are summarized in Table 1 (41-45).
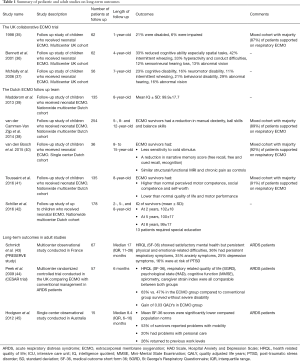
Full table
Prolonged ECMO runs
Many clinicians will consider a prolonged ECMO run as one in which the duration of support is longer than 21 days (46). While there is no absolute cut-off for ECMO support, the clinician must be aware of the shifting risk-benefit balance in patients requiring a prolonged ECMO course. The longest run time in pediatric respiratory ECMO prior to year 2000 was 62 days, but over the last decade, there are increasing reports of longer and longer ECMO runs (up to 129 days) (1). In a recent ELSO review, 439 neonates were supported with prolonged ECMO (>21 days) (46). The majority [300/439 (68%)] of patients had >3 complications. The most common complications were mechanical complications from the ECMO circuitry [346/439 (79%)] and hemorrhage [218/439 (50%)] (46). Among this group who required prolonged ECMO support, the need for inotropic support was independently associated with mortality. The long-term consequences of a prolonged ECMO run should be considered in the daily management of patients on ECMO and during counselling of parents.
Novel management strategies
Advancement in techniques and technology
Traditionally, VV ECMO was often achieved by cannulation of the internal jugular and femoral veins. A dual lumen cannula, first described in adults in 2010 has been increasingly utilized in children (47). This cannula (Avalon LLC, Maquet, Rastatt, Germany) draws deoxygenated blood from the superior and inferior vena cava while directing oxygenated blood toward the tricuspid valve into the right ventricle. Refinements in the design of this cannula have provided opportunities in the neonatal and pediatric population (48,49).
A single centre retrospective study (n=72) reported the use of dual lumen cannulas in a cohort of neonates with a median weight of 3.4 (interquartile range, 3.0–3.7) kg (50). Another case series (n=19) reported successful percutaneous placement in neonates as small as 2.5 kg (51). Despite more frequent cannula problems in 22.3% and cardiovascular problems (including all cause cardiac tamponade) in 24.4%, there is increasing usage of the dual lumen cannula surpassing the use of multisite single lumen cannulas in 2001 and doubling in numbers in 2011 (52). The use of dual lumen cannulas has also been associated with a survival benefit compared to single lumen cannulas [OR, 1.95 (95% CI, 1.24–3.07)] (52).
Cannulation techniques have also evolved. Safe percutaneous insertion of venous cannulas by intensivists has been reported for both dual lumen and single lumen multisite configurations (30,53). Flows generated from this technique were adequate and cannulation times reportedly as low as 25–30 minutes (54). Percutaneous insertion of arterial cannulas has also been reported, but the femoral route was more common compared to the carotid route (30,53). Other innovative cannulation techniques such as percutaneous transhepatic cannulation of the right hepatic vein for VV ECMO have also been described (55).
Early mobilisation and rehabilitation
Pediatric patients on ECMO have traditionally been maintained deeply sedated and, at times, paralyzed. Excess sedation and unnecessary paralysis have been recognized as risk factors for increased morbidity and mortality in the critically ill population (56,57). There has been a recent paradigm shift within the ICU community toward the need for less sedation and earlier mobilization of critically ill patients (58-60). While there is a potential risk of dislodgement of cannulas and ECMO circuit components with mobilizing patients on ECMO, there are an increasing number of studies that have demonstrated that with careful logistic planning, sedation can be minimized and patients mobilized even while being supported on ECMO (61-63). Although this concern of dislodgement is theoretically higher in children, there are emerging studies to demonstrate that awake ECMO can be safely utilized in children.
The first description of three children (10–14 years old) supported on VV ECMO (duration 12–109 days) while awaiting lung transplantation demonstrated the feasibility of awake ECMO in the pediatric population (64). The specific subset of patients in which awake ECMO had been studied and described is in the pre-lung transplant population. Because this group of patients has usually adapted to the chronicity of their respiratory illness and reserve, clinicians need to be careful of extrapolating these data to patients with ARDS, in whom the majority were previously healthy. As such, these patients may not be able to tolerate the lack of support of mechanical ventilation and the resultant marked tachypnea while supported on ECMO (65). However, a recent report demonstrated that an approach of ambulatory ECMO for an ARDS patient can be successful (66). In this case report, the 16-year-old patient underwent a tracheostomy to facilitate rehabilitation while awaiting lung transplantation. Another retrospective study demonstrated feasibility of extubation while on ECMO—14/16 (88%) of these were respiratory cases and of these 5/14 (36%) were neonates (67). The rationale was that prolonged ECMO runs were becoming the norm and patients were being exposed to greater sedation toxicity. In this study, extubated patients required less sedation, spent more time at a State Behavioural Scale of 0 and were more active/interactive (67). No direct complications of extubation were reported.
In contrast, there is more experience described for “awake ECMO” in adult patients. ECMO cannulation can be performed under local anaesthesia, and the entire ECMO run carried out in a fully conscious adult for prolonged periods of time (2–5 weeks) while awaiting lung transplantation or potentially lung recovery (68). During this time, the patient is breathing spontaneously, eating, drinking, receiving physiotherapy and psychological support (68). Data from a retrospective study (n=11) showed a reduction in critical illness myopathy and polyneuropathy in the “awake ECMO” group compared to the mechanically ventilated group (42.8% vs. 100%, P=0.04) (69). Another study (n=60) demonstrated that patients on “awake ECMO” had a shorter post-operative duration of mechanical ventilation compared patients ventilated on ECMO (14 vs. 37 days, P=0.04) (63).
Liberation from the mechanical ventilation facilitates active physiotherapy and ambulation, and can be safely achieved (70). Technical considerations as well as pros and cons of “awake ECMO” are beyond the scope of this review, and we refer the readers to an excellent review of this topic (71). It is likely that “awake ECMO” and early mobilisation will become the new standard of care for patients bridging to lung transplant and potentially for those bridging to recovery (72).
Future direction
As the medical community becomes more familiar with this support modality, the traditional relative and absolute contraindications to ECMO are being challenged. There are scattered case reports of successful respiratory ECMO runs in children with increasingly complex pathologies and co-morbidities (27,73,74). The utility of concurrent medical therapies (e.g., immunotherapy, plasmapheresis, molecular adsorbent recirculating system dialysis) are increasingly being utilized (75-77). Despite this increasing list of successful case reports, careful patient selection remains the most important factor to be considered by ECMO providers.
ECMO should optimally be reserved for self-limited/reversible disease processes with occasional use as a “bridge” to definitive treatment (e.g., lung and/or heart transplantation) and rare use in acutely deteriorating patients in whom reversibility cannot be quickly and accurately determined (“bridge to decision”). In addition to assessing the severity and reversibility of the lung injury, other clinical factors such as presence of significant co-morbidities, genetic syndromes, neurological status, and concomitant organ dysfunction should be considered.
The concept of utilizing ECMO to avoid ventilator-induced lung injury is not a recent one (78). Indeed, the landmark study conducted by the ARDS Network demonstrated that 6 mL/kg ideal body weight of tidal volume ventilation offered significant survival benefit in patients with ARDS compared to 12 mL/kg prescription of mechanical ventilation (79). However, what remains uncertain is the ideal tidal volume that patients with ARDS require. This raises the possibility that perhaps an even lower tidal volume strategy may offer more optimal lung rest. In one of the first studies to examine this issue, the Xtravent-study compared the utility of a lower tidal volume strategy (3 mL/kg) combined with extracorporeal CO2 removal against the conventionally accepted lung protective ventilation strategy of 6 mL/kg in adults with ARDS (80). In this study of 79 adult patients with ARDS, there was no difference in the primary outcomes of interest with no difference in mean 28-day and 60-day ventilator-free days between those supported on lower tidal volume ventilation as compared to a conventional 6 mL/kg strategy (10.0±8 vs. 9.3±9, P =0.779 and 33.0±20 vs. 29.2±21, P =0.469, respectively). While this study did not specifically utilize VV ECMO in the protocol, the concept of utilizing an extracorporeal circuit to assist in mechanical ventilation while pushing the lower limit of tidal volume ventilation is a novel one. The possibility of potential lung derecruitment from the use of lower tidal volumes should be borne in mind. Nevertheless, the stage is set for future studies to examine the utility of ECMO in pushing the lower limit of tidal volume ventilation and the optimal prescription of lung rest in severe lung injury. Pediatric guidelines or recommendations on ventilation strategy while on ECMO are currently not available, though experts agree that it is important to minimize ventilator induced lung injury (81). This can be done by maintaining lung recruitment with positive end expiratory pressure and limiting peak/plateau pressures and tidal volumes, thus limiting risk for air leak (81,82). Gas exchange is maintained by the ECMO circuit.
The most important, yet challenging, consideration for the bedside clinician is patient selection and timing of transitioning to and from ECMO support. Areas for future research in the utility of ECMO in pediatric severe respiratory failure include inclusion/exclusion criteria, optimal “lung rest” ventilator settings, efficacy of adjunct therapies, timing of cannulation, effects of ambulation, and long-term outcome data including respiratory, cardiac, and neurologic functioning for all pediatric survivors of respiratory ECMO.
Conclusions
Studies on ECMO are mostly retrospective from the ELSO registry and, thus, constitute low level evidence. However, over the past few years, there has been a dramatic increase in the number of studies reporting changing trends in use and evolving techniques and technologies. Among these reports, we observed that indications for respiratory ECMO are growing beyond its traditional use for pulmonary parenchymal disease. Patients with large and small airway diseases refractory to conventional management are increasingly being supported on ECMO. In addition, contraindications that were previously considered as absolute are now becoming relative contraindications. Increasingly complex medical pediatric patients with severe respiratory failure will continue to be supported on ECMO. The presence of complex comorbidities may potentially impact of the overall survival of children supported on respiratory ECMO. Clinicians examining outcome data of pediatric respiratory ECMO should be mindful of this changing epidemiology of this cohort of children. The lack of change in survival figures over time may be a reflection of the increasingly complex patients who are supported with ECMO instead of the lack of improvement of ECMO management. Indeed, there has been steady advancement in the field of neonatal and pediatric ECMO as evident by the advent of dual lumen venous cannulas, percutaneous cannulation techniques, and awake/ambulatory ECMO within the last decade.
The application of ECMO for neonatal/pediatric respiratory failure is likely to continue to increase given the broadening indications and continual development of innovations in technology. Children supported on respiratory ECMO are expected to become more complex and co-morbidities once considered as contraindications will be challenged. Swifter and safer cannulation techniques by percutaneous means will likely contribute to increased usage. Given this changing landscape, it will be more important than ever for the critical care provider to refine patient selection and determine the optimal time for ECMO cannulation. Over the next five years, with the expectant advancement in invasive mechanical ventilation support, one of the most challenging decisions for the clinician will remain patient selection and timing of ECMO support. Prolonged ECMO runs are expected to become increasingly more common. Efforts to minimize long-term morbidities and optimize functional outcomes in pediatric respiratory ECMO survivors will be important. Further studies are essential to refine the management of children supported on ECMO. Additional data are needed to facilitate appropriate sedation in children supported on ECMO while at the same time, allowing these children, when appropriate, to be awake, extubated and ambulatory.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- International Summary. ECLS Registry Report. Extracorporeal Life Support Organization. January 2016.
- Norfolk SG, Hollingsworth CL, Wolfe CR, et al. Rescue therapy in adult and pediatric patients with pH1N1 influenza infection: a tertiary center intensive care unit experience from April to October 2009. Crit Care Med 2010;38:2103-7. [Crossref] [PubMed]
- Turner DA, Rehder KJ, Peterson-Carmichael SL, et al. Extracorporeal membrane oxygenation for severe refractory respiratory failure secondary to 2009 H1N1 influenza A. Respir Care 2011;56:941-6. [Crossref] [PubMed]
- Hill JD, O'Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med 1972;286:629-34. [Crossref] [PubMed]
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979;242:2193-6. [Crossref] [PubMed]
- Bartlett RH, Gazzaniga AB, Huxtable RF, et al. Extracorporeal circulation (ECMO) in neonatal respiratory failure. J Thorac Cardiovasc Surg 1977;74:826-33. [PubMed]
- O'Rourke PP, Crone RK, Vacanti JP, et al. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: a prospective randomized study. Pediatrics 1989;84:957-63. [PubMed]
- UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. UK Collaborative ECMO Trail Group. Lancet 1996;348:75-82. [Crossref] [PubMed]
- Steinhorn RH, Green TP. Use of extracorporeal membrane oxygenation in the treatment of respiratory syncytial virus bronchiolitis: the national experience, 1983 to 1988. J Pediatr 1990;116:338-42. [Crossref] [PubMed]
- Scalzo AJ, Weber TR, Jaeger RW, et al. Extracorporeal membrane oxygenation for hydrocarbon aspiration. Am J Dis Child 1990;144:867-71. [PubMed]
- Extracorporeal Life Support Organization (ELSO). Extracorporeal Life Support Registry Report International Summary. Ann Arbor, MI: ELSO; 2013.
- Norfolk SG, Hollingsworth CL, Wolfe CR, et al. Rescue Therapy in Adult and Pediatric Patients with H1N1 Influenza Infection: A Tertiary Center ICU Experience from April-October, 2009. Crit Care Med 2010;38:2103-7. [Crossref] [PubMed]
- Rehder KJ, Turner DA, Cheifetz IM. Extracorporeal Membrane Oxygenation for Neonatal and Pediatric Respiratory Failure: An Evidence-Based Review of the Past Decade (2002-2012). Pediatr Crit Care Med 2013;14:851-61. [Crossref] [PubMed]
- Huang YL, Yang MC, Huang CH, et al. Rescue of cardiopulmonary collapse in anterior mediastinal tumor: case presentation and review of literature. Pediatr Emerg Care 2010;26:296-8. [Crossref] [PubMed]
- Hebbar KB, Petrillo-Albarano T, Coto-Puckett W, et al. Experience with use of extracorporeal life support for severe refractory status asthmaticus in children. Crit Care 2009;13:R29. [Crossref] [PubMed]
- Baqais KA, Mahoney M, Tobler K, et al. Pediatric sand aspiration managed using bronchoscopy and extracorporeal membrane oxygenation. Can Respir J 2015;22:261-2. [Crossref] [PubMed]
- Panarello G, Occhipinti G, Piazza M, et al. Severe Acute Respiratory Failure due to Inhalation of Baby Powder and Successfully Treated with Venous-Venous Extracorporeal Membrane Oxygenation. A A Case Rep 2015;5:228-30. [Crossref] [PubMed]
- Halasa NB, Barr FE, Johnson JE, et al. Fatal pulmonary hypertension associated with pertussis in infants: does extracorporeal membrane oxygenation have a role? Pediatrics 2003;112:1274-8. [Crossref] [PubMed]
- Zabrocki LA, Brogan TV, Statler KD, et al. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med 2011;39:364-70. [Crossref] [PubMed]
- O'Rourke PP, Stolar CJ, Zwischenberger JB, et al. Extracorporeal membrane oxygenation: support for overwhelming pulmonary failure in the pediatric population. Collective experience from the extracorporeal life support organization. J Pediatr Surg 1993;28:523-8; discussion 528-9. [Crossref] [PubMed]
- Gow KW, Heiss KF, Wulkan ML, et al. Extracorporeal life support for support of children with malignancy and respiratory or cardiac failure: The extracorporeal life support experience. Crit Care Med 2009;37:1308-16. [Crossref] [PubMed]
- Gupta M, Shanley TP, Moler FW. Extracorporeal life support for severe respiratory failure in children with immune compromised conditions. Pediatr Crit Care Med 2008;9:380-5. [Crossref] [PubMed]
- Di Nardo M, Locatelli F, Palmer K, et al. Extracorporeal membrane oxygenation in pediatric recipients of hematopoietic stem cell transplantation: an updated analysis of the Extracorporeal Life Support Organization experience. Intensive Care Med 2014;40:754-6. [PubMed]
- Cashen K, Thiagarajan RR, Collins JW Jr, et al. Extracorporeal Membrane Oxygenation in Pediatric Trisomy 21: 30 Years of Experience from the Extracorporeal Life Support Organization Registry. J Pediatr 2015;167:403-8. [Crossref] [PubMed]
- Riojas C, Pipkin W. Initiation of ECMO for ventilator-dependent respiratory failure in an infant with Pompe's. Pediatr Surg Int 2014;30:553-5. [Crossref] [PubMed]
- Frey TK, Chopra A, Lin RJ, et al. A child with anterior mediastinal mass supported with veno-arterial extracorporeal membrane oxygenation. Pediatr Crit Care Med 2006;7:479-81. [Crossref] [PubMed]
- Pooboni S, Roberts N, Westrope C, et al. Extracorporeal life support in pertussis. Pediatr Pulmonol 2003;36:310-5. [Crossref] [PubMed]
- Paden ML, Rycus PT, Thiagarajan RR. Update and outcomes in extracorporeal life support. Semin Perinatol 2014;38:65-70. [Crossref] [PubMed]
- Guner YS, Khemani RG, Qureshi FG, et al. Outcome analysis of neonates with congenital diaphragmatic hernia treated with venovenous vs venoarterial extracorporeal membrane oxygenation. J Pediatr Surg 2009;44:1691-701. [Crossref] [PubMed]
- Rollins MD, Hubbard A, Zabrocki L, et al. Extracorporeal membrane oxygenation cannulation trends for pediatric respiratory failure and central nervous system injury. J Pediatr Surg 2012;47:68-75. [Crossref] [PubMed]
- Pranikoff T, Hirschl RB, Steimle CN, et al. Mortality is directly related to the duration of mechanical ventilation before the initiation of extracorporeal life support for severe respiratory failure. Crit Care Med 1997;25:28-32. [Crossref] [PubMed]
- Domico MB, Ridout DA, Bronicki R, et al. The impact of mechanical ventilation time before initiation of extracorporeal life support on survival in pediatric respiratory failure: a review of the Extracorporeal Life Support Registry. Pediatr Crit Care Med 2012;13:16-21. [Crossref] [PubMed]
- Jen HC, Shew SB. Hospital readmissions and survival after nonneonatal pediatric ECMO. Pediatrics 2010;125:1217-23. [Crossref] [PubMed]
- Taylor AK, Cousins R, Butt WW. The long-term outcome of children managed with extracorporeal life support: an institutional experience. Crit Care Resusc 2007;9:172-7. [PubMed]
- The collaborative UK ECMO (Extracorporeal Membrane Oxygenation) trial. follow-up to 1 year of age. Pediatrics 1998;101:E1. [PubMed]
- Bennett CC, Johnson A, Field DJ, et al. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation: follow-up to age 4 years. Lancet 2001;357:1094-6. [Crossref] [PubMed]
- McNally H, Bennett CC, Elbourne D, et al. United Kingdom collaborative randomized trial of neonatal extracorporeal membrane oxygenation: follow-up to age 7 years. Pediatrics 2006;117:e845-54. [Crossref] [PubMed]
- van der Cammen-van Zijp MH, Janssen AJ, Raets MM, et al. Motor performance after neonatal extracorporeal membrane oxygenation: a longitudinal evaluation. Pediatrics 2014;134:e427-35. [Crossref] [PubMed]
- Madderom MJ, Reuser JJ, Utens EM, et al. Neurodevelopmental, educational and behavioral outcome at 8 years after neonatal ECMO: a nationwide multicenter study. Intensive Care Med 2013;39:1584-93. [Crossref] [PubMed]
- van den Bosch GE, IJsselstijn H, van der Lugt A, et al. Neuroimaging, Pain Sensitivity, and Neuropsychological Functioning in School-Age Neonatal Extracorporeal Membrane Oxygenation Survivors Exposed to Opioids and Sedatives. Pediatr Crit Care Med 2015;16:652-62. [Crossref] [PubMed]
- Toussaint LC, van der Cammen-van Zijp MH, Janssen AJ, et al. Perceived Motor Competence Differs From Actual Performance in 8-Year-Old Neonatal ECMO Survivors. Pediatrics 2016;137:e20152724. [Crossref] [PubMed]
- Schiller RM, Madderom MJ, Reuser JJ, et al. Neuropsychological Follow-up After Neonatal ECMO. Pediatrics 2016.138. [PubMed]
- Schmidt M, Zogheib E, Rozé H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013;39:1704-13. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Hodgson CL, Hayes K, Everard T, et al. Long-term quality of life in patients with acute respiratory distress syndrome requiring extracorporeal membrane oxygenation for refractory hypoxaemia. Critical Care 2012;16:R202. [Crossref] [PubMed]
- Prodhan P, Stroud M, El-Hassan N, et al. Prolonged extracorporeal membrane oxygenator support among neonates with acute respiratory failure: a review of the Extracorporeal Life Support Organization registry. ASAIO J 2014;60:63-9. [Crossref] [PubMed]
- Bermudez CA, Rocha RV, Sappington PL, et al. Initial experience with single cannulation for venovenous extracorporeal oxygenation in adults. Ann Thorac Surg 2010;90:991-5. [Crossref] [PubMed]
- Lazar DA, Cass DL, Olutoye OO, et al. Venovenous cannulation for extracorporeal membrane oxygenation using a bicaval dual-lumen catheter in neonates. J Pediatr Surg 2012;47:430-4. [Crossref] [PubMed]
- Fallon SC, Shekerdemian LS, Olutoye OO, et al. Initial experience with single-vessel cannulation for venovenous extracorporeal membrane oxygenation in pediatric respiratory failure. Pediatr Crit Care Med 2013;14:366-73. [Crossref] [PubMed]
- Speggiorin S, Robinson SG, Harvey C, et al. Experience with the Avalon(R) bicaval double-lumen veno-venous cannula for neonatal respiratory ECMO. Perfusion 2015;30:250-4. [Crossref] [PubMed]
- Czerwonko ME, Fraga MV, Goldberg DJ, et al. Cardiovascular perforation during placement of an Avalon Elite(R) Bicaval dual lumen ECMO cannula in a newborn. J Card Surg 2015;30:370-2. [Crossref] [PubMed]
- Zamora IJ, Shekerdemian L, Fallon SC, et al. Outcomes comparing dual-lumen to multisite venovenous ECMO in the pediatric population: the Extracorporeal Life Support Registry experience. J Pediatr Surg 2014;49:1452-7. [Crossref] [PubMed]
- Conrad SA, Grier LR, Scott LK, et al. Percutaneous cannulation for extracorporeal membrane oxygenation by intensivists: a retrospective single-institution case series. Crit Care Med 2015;43:1010-5. [Crossref] [PubMed]
- Moscatelli A, Buratti S, Gregoretti C, et al. Emergency percutaneous, bicaval double-lumen, ECMO cannulation in neonates and infants: insights from three consecutive cases. Int J Artif Organs 2015;38:517-21. [Crossref] [PubMed]
- Rhodes LA, Sasser WC, McMahon WS, et al. Transhepatic Cannulation for Venovenous Extracorporeal Membrane Oxygenation. ASAIO J 2015;61:e29-30. [Crossref] [PubMed]
- Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med 2012;186:724-31. [Crossref] [PubMed]
- Shehabi Y, Chan L, Kadiman S, et al. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med 2013;39:910-8. [Crossref] [PubMed]
- Shehabi Y, Bellomo R, Reade MC, et al. Early goal-directed sedation versus standard sedation in mechanically ventilated critically ill patients: a pilot study*. Crit Care Med 2013;41:1983-91. [Crossref] [PubMed]
- Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med 2008;36:2238-43. [Crossref] [PubMed]
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874-82. [Crossref] [PubMed]
- Turner DA, Cheifetz IM, Rehder KJ, et al. Active rehabilitation and physical therapy during extracorporeal membrane oxygenation while awaiting lung transplantation: a practical approach. Crit Care Med 2011;39:2593-8. [Crossref] [PubMed]
- Rehder KJ, Turner DA, Hartwig MG, et al. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir Care 2013;58:1291-8. [Crossref] [PubMed]
- Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012;185:763-8. [Crossref] [PubMed]
- Schmidt F, Sasse M, Boehne M, et al. Concept of "awake venovenous extracorporeal membrane oxygenation" in pediatric patients awaiting lung transplantation. Pediatr Transplant 2013;17:224-30. [Crossref] [PubMed]
- Cheifetz IM. Extracorporeal membrane oxygenation of the future: smaller, simpler, and mobile. Pediatr Transplant 2013;17:202-4. [Crossref] [PubMed]
- Turner DA, Rehder KJ, Bonadonna D, et al. Ambulatory ECMO as a Bridge to Lung Transplant in a Previously Well Pediatric Patient with ARDS. Pediatrics 2014;134:e583-5. [Crossref] [PubMed]
- Anton-Martin P, Thompson MT, Sheeran PD, et al. Extubation during pediatric extracorporeal membrane oxygenation: a single-center experience. Pediatr Crit Care Med 2014;15:861-9. [Crossref] [PubMed]
- Olsson KM, Simon A, Strueber M, et al. Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am J Transplant 2010;10:2173-8. [Crossref] [PubMed]
- Nosotti M, Rosso L, Tosi D, et al. Extracorporeal membrane oxygenation with spontaneous breathing as a bridge to lung transplantation. Interact Cardiovasc Thorac Surg 2013;16:55-9. [Crossref] [PubMed]
- Abrams D, Javidfar J, Farrand E, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care 2014;18:R38. [Crossref] [PubMed]
- Langer T, Santini A, Bottino N, et al. "Awake" extracorporeal membrane oxygenation (ECMO): pathophysiology, technical considerations, and clinical pioneering. Crit Care 2016;20:150. [Crossref] [PubMed]
- MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 2012;38:210-20. [Crossref] [PubMed]
- Gandhi NN, Hartman ME, Williford WL, et al. Successful use of extracorporeal membrane oxygenation for pH1N1-induced refractory hypoxemia in a child with hypoplastic left heart syndrome. Pediatr Crit Care Med 2011;12:e398-401. [Crossref] [PubMed]
- Ballouhey Q, Fesseau R, Benouaich V, et al. Benefits of extracorporeal membrane oxygenation for major blunt tracheobronchial trauma in the paediatric age group. Eur J Cardiothorac Surg 2013;43:864-5. [Crossref] [PubMed]
- Tabata S, Cavarocchi NC, Hirose H. Successful management of severe liver failure on venoarterial extracorporeal membrane oxygenation using molecular adsorbent recirculating systeme. J Heart Lung Transplant 2012;31:1322-3. [Crossref] [PubMed]
- Keller R, Torres S, Iölster T, et al. Extracorporeal membrane oxygenation and plasmapheresis in the treatment of severe pulmonary hemorrhage secondary to nodose polyarteritis. Arch Argent Pediatr 2012;110:e80-5. [Crossref] [PubMed]
- Di Nardo M, Li Pira G, Amodeo A, et al. Adoptive immunotherapy with antigen-specific T cells during extracorporeal membrane oxygenation (ECMO) for adenovirus-related respiratory failure in a child given haploidentical stem cell transplantation. Pediatr Blood Cancer 2014;61:376-9. [Crossref] [PubMed]
- Gattinoni L, Agostoni A, Pesenti A, et al. Treatment of acute respiratory failure with low-frequency positive-pressure ventilation and extracorporeal removal of CO2. Lancet 1980;2:292-4. [Crossref] [PubMed]
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Bein T, Weber-Carstens S, Goldmann A, et al. Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus 'conventional' protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med 2013;39:847-56. [Crossref] [PubMed]
- Maslach-Hubbard A, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: History, development and current status. World J Crit Care Med 2013;2:29-39. [Crossref] [PubMed]
- Schmidt M, Pellegrino V, Combes A, et al. Mechanical ventilation during extracorporeal membrane oxygenation. Critical Care 2014;18:203. [Crossref] [PubMed]
Cite this article as: Wong JJ, Cheifetz IM, Lee JH. Extracorporeal membrane oxygenation for severe pediatric respiratory failure. J Emerg Crit Care Med 2017;1:11.


