Before and after standardizing the controversial sepsis resuscitation bundle in a large hybrid academic center
Introduction
Sepsis is a life-threatening disease associated with a high mortality and cost. As such, early recognition and intervention is paramount in improving outcomes (1). The Surviving Sepsis Campaign (SSC) provides best practice guidelines for the management of severe sepsis and septic shock. SSC was initially created in 2002 after a consensus committee of international experts met, motivated by the landmark trial by Dr. Rivers et al. (2) which encouraged the implementation of the sepsis bundle of care to improve in-hospital mortality. Subsequent recommendations have reiterated the creation of sepsis bundles with concordant performance measures (3). More recent studies also validated that implementation of sepsis bundle reduces mortality (4). However, a 2012 SSC prospective cohort study revealed compliance with recommendations have been poor and there is considerable variability in treatment (5). Several hospital systems have resorted to using automated detection approach to alert for the possibility of sepsis. However, the care team alerts did not consistently translate to earlier interventions or improved outcomes (6). While the interventions recommended in the Rivers trial have recently been shown not to be beneficial in severe sepsis, the creation of the sepsis bundle and involvement of several providers in the healthcare system continues to be recommended (7).
We hypothesized that a structured protocol consisting of a modified sepsis bundle linked to an automated best practice alert (BPA) as well as coordination of care between emergency and intensive care providers would improve early recognition and treatment initiation for patients presenting to the ED with severe sepsis or septic shock. In addition, we felt this would streamline admissions to the ICU setting when appropriate and decrease in-hospital mortality. We utilize the terms “sepsis”, “severe sepsis”, and “septic shock” based on the 2012 SSC guidelines to provide consistency with existing literature at the time, rather than Sepsis 3.0 definition which was updated in 2017 (8).
Methods
Study population
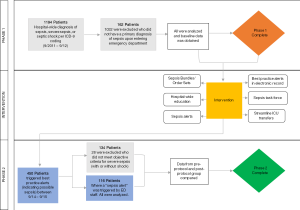
Our study was divided into two phases (Figure 1). The first phase involved a retrospective chart review to obtain baseline data. We utilized administrative data to identify patients admitted between September 1, 2011 and September 1, 2012 through the emergency department (ED) with a primary discharge ICD-9 diagnosis of “sepsis”, “severe sepsis” or “septic shock”. Baseline data was collected on all patients who met the following criteria: age 18 or older and primary diagnosis of sepsis when presenting to the ED. Sepsis was defined as per the SSC as evidence of a source infection occurring accompanied by at least two of the following SIRS criteria: (I) temp >38 °C (100.4 °F) or <36 °C (96.8 °F); (II) heart rate >90; (III) respiratory rate >20 or PaCO2 <33 mmHg; (IV) WBC >12,999/mm3, <4,000/mm3, or >10% bands.
Patients with objective evidence of end organ dysfunction upon presentation to the ED with sepsis (i.e., those with severe sepsis +/− shock) were included as the final baseline comparison group. Signs of end organ damage included: (I) urine output <0.5 mg/kg/h for >2 hours or serum creatinine >2 mg/dL; (II) new onset altered mental status; (III) SBP <90 mmHg or MAP <65 mmHg; (IV) bilirubin >2 mg/dL; (V) platelet count <100,000/mm3; (VI) lactate >2 mmol/L.
In phase 2, after the new protocol implementation, patient charts were included in the review if they triggered the BPA indicating possible sepsis in the ED. Patients in which the ED provider activated the “sepsis” alert were analyzed. Those meeting the criteria for “severe sepsis” with or without shock were compared to the baseline group.
The study was conducted in a 560-bed hybrid medical staff hospital consisting of both community and academic providers. The protocol was reviewed and approved by the University of Miami Institutional Review Board (ID 20120298).
Protocol development and implementation
We convened a multidisciplinary sepsis task force comprised of attending physicians (ICU, ED, and infectious disease), house staff, nurses, nursing educators, pharmacy, and laboratory leadership in response to the data obtained from phase 1 of the study. The task force was responsible for initiating in 2012 a quality improvement intervention at our institution to address the patients presenting with sepsis to the ED. The taskforce reviewed clinically relevant literature and developed a mandatory sepsis order set based on the 2012 SSC guideline recommendations (9) to standardize the approach to resuscitation. Antibiotics options were based on the Infectious Diseases Society of America (IDSA) Practice Guidelines for the following suspected sites of infection: urine, soft tissue/skin, febrile neutropenia, community acquired pneumonia, hospital acquired pneumonia, intra-abdominal sources and unknown sources (10). In addition, they worked with IT to create a BPA in the electronic medical record (EMR), the goal of which was to facilitate early recognition of sepsis in the ED. The BPA was set to trigger if at least two SIRS criteria were met on initial vitals (see criteria above) and was linked to the new sepsis order set. The taskforce launched a house-wide educational campaign over the next 6 months focused on early recognition and intervention for patients with sepsis based on the SSC guideline recommendations of 2012. The education started with a formal presentation to nurses in January 2013, followed by multiple in-service didactics to nurses and physicians outlining the importance of early recognition and treatment of sepsis and the timing of bundle implementation. Although our study focused on patients who presented to the ED with sepsis, education was delivered to all departments to help recognize and identify all patients who exhibited signs of sepsis during their hospitalization. This education was reinforced to clinicians, nurses, and house staff with a particular focus on the identification of severe sepsis and septic shock patients presenting via the ED. Badge buddies outlining the protocol and the expected interventions were provided during the educational series.
Protocol
The new protocol is outlined in Figure 2. Patients presenting to the ED exhibiting at least two SIRS criteria in the initial ED assessment triggered the BPA. The ED physician was notified and had the option to accept the alert if an infection was suspected. If the BPA was accepted, the ED physician was prompted to initiate a sepsis bundle of resuscitation. The initial sepsis bundle included the following: (I) large bore peripheral IV; (II) fluids of 0.9% normal saline; (III) lab draws (including lactic acid and blood cultures); (IV) initiation of antibiotics after cultures. For patients with severe sepsis or septic shock, the ED physician activates a “sepsis alert” via the page operator, notifying pharmacy and the sepsis alert team (MICU fellow or ARNP, ICU bed control, nursing supervisor) of the patient. The MICU clinician responds to the ED, initiates the severe sepsis admission order set and initiates transfer to the ICU if appropriate.
Data collection for phase 2 of the study began after the protocol had been in place for over 6 months in order to permit the full implementation of all components of the sepsis alert bundle and to allow any initial deployment hurdles to be identified and addressed. Starting in September 2014, we reviewed all patients who presented to the ED in which the ED physician activated the sepsis alert (i.e., those with severe sepsis or septic shock). Data collected included time from initial vitals to initiation of cultures, fluids and antibiotics, sequential organ failure assessment (SOFA) score (11), length of stay (LOS) in the hospital, and in-hospital mortality. We obtained the same data from the baseline patients (phase 1) and compared the results to post-implementation patients (phase 2) to assess impact.
Analytic plan
Descriptive statistics were calculated and distributions were examined. Continuous measures were compared using a t-test for independent samples. Chi-square tests were used to compare proportions of categorical data, using statistical significance level of P<0.05. Among analyses using a t-test, if Levene’s test for equality of variances was statistically significant indicating unequal variances, we used the corrected estimate for the error term for the t-statistic and the adjusted degrees of freedom using the Welch-Satterthwaite method. For analyses adjusting for covariates, linear regressions were used to continuous outcomes and logistic regressions were used for categorical outcomes. Predictors (before/after bundle implementation, elapsed time to each component of treatment) were used to determine outcomes (mortality, time to treatment components, LOS) after adjusting for age, sex, and sepsis severity as covariates. Data were analyzed using SPSS Version 22.0.0.2 (IBM). None of the patients included in the analyses were lost due to follow up in that all were followed to discharge.
Results
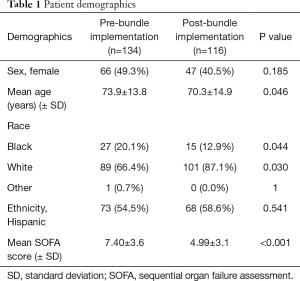
Prior to the bundle implementation, 1,194 patients in hospital-wide were discharged between September 2011 and September 2012 with an ICD-9 diagnosis of sepsis, severe sepsis, or septic shock (Figure 1). A total of 1,032 were initially excluded as they did not meet the criteria for sepsis when they presented to the ED. We collected baseline data on the remaining 162 patients for phase 1 of the trial. 134 of these met criteria for severe sepsis or septic shock and were included in the final analysis as the baseline comparison group. The mean age was 73.9 years [standard deviation (SD) =13.8 years] (Table 1). The combined inpatient mortality rate for all categories of sepsis was 32% while severe sepsis and septic shock was 41.8%. Average LOS was 11.2±1.3 days. The SOFA score for those who had expired was significantly higher than those alive at discharge {8.47 vs. 5.95, t[152]=−4.099, P<0.001} (Table 1). Multiple linear regression analyses revealed that SOFA scores were not predictive of timing to order or delivery of antibiotics, fluids, or blood cultures even after controlling for age and gender (P>0.05). There was a large variance in treatment time: differences in mean time (minutes) to blood culture collection (alive: 59.78; expired: 96.20), antibiotic administration (alive: 201.69; expired: 236.55), and fluid administration (alive: 321.38; expired: 1,772.15) were accompanied by vastly different SDs in treatment timings [blood culture SD: alive =591.79 min, expired =645.31 min; antibiotics SD: alive =252.61 min, expired =254.34 min; fluids (SD): alive =1,604.76 min, expired =7,573.20 min].
Full table
After the bundle implementation and education, 450 BPAs were triggered over one year between September 2014 and 2015, indicating a possible diagnosis of sepsis upon presentation to the ED. In 116 patients, ED providers triggered a “Sepsis Alert” (indicating a diagnosis of severe sepsis or septic shock) and all patients were analyzed.
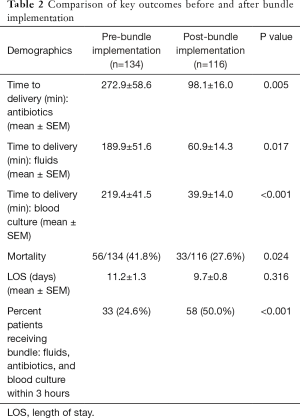
We then compared results between phase 1 and 2 (Table 2). Prior to bundle implementation, mortality in severe sepsis was 56/134 (41.8%) and decreased to 32/116 {27.6%; χ2[1]=5.50, P=0.024}. Since sepsis type directly impacted mortality as an outcome, we then adjusted for sepsis type along with age and gender to determine the effect of bundle implementation on mortality. After controlling for age, gender, and sepsis type (sepsis vs. severe sepsis/septic shock), patients were more than twice as likely to survive after the implementation of the sepsis bundle (OR =2.109, 95% CI: 1.196–3.719, P=0.010).
Full table
In addition to improving mortality, sepsis bundle implementation was found to significantly improve the percent of individuals who received key elements of the bundle (blood culture, fluids, and antibiotics) within 3 hours of arrival (Table 2). In total, 50.0% were treated within 3 hours of arrival post-implementation as compared to 24.6% prior to implementation (P<0.001). LOS did not differ before and after bundle implementation {pre: 11.2±1.3 days, post: 9.7±0.8 days; t[247]=1.00, P=0.316}.
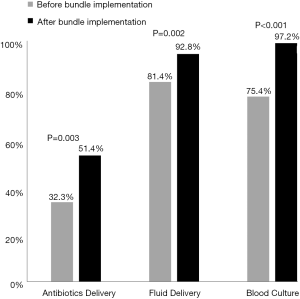
We compared key treatment components before and after bundle implementation (Figure 3), namely delivery of antibiotics, delivery of fluids, and performing a blood culture, to determine any significant changes in the proportion of patients for whom treatment was received within the times suggested by the sepsis guidelines. Specifically, compliance occurs if each treatment component is received within three hours from initial vitals; one hour is the cutoff for antibiotics delivery for those with severe sepsis. There was a significant increase in the proportion of patients receiving antibiotics within the recommended time {pre: 32.3%, post: 51.4%; χ2[1]=4.83, P=0.003}, fluids {pre: 81.4%, post: 92.8%; χ2[1]=6.59, P=0.012}, and a blood culture {pre: 75.4%, post: 97.2%; χ2[1]=22.91, P<0.001}.
Discussion
Sepsis is a life-threatening dynamic disease that requires close monitoring while assessing response to treatment. Standardizing care via established protocols remains a topic of debate with recent critique in ARISE and ProCESS (12,13) trials showing no change in outcome between early goal-directed therapy (EGDT) versus usual care (7). However, in our facility we found improved outcomes after sepsis order sets linked to a BPA were established and implemented and care was standardized. Our protocol was based on the 2012 SSC guidelines, which emphasizes a global patient assessment to detect sepsis as well as the utilization of time-sensitive treatment bundles. It is estimated that half of sepsis presentations occur in the ED (14,15) making this a key area for implementing bundles. By improving early sepsis recognition and facilitating immediate transfer to the ICU when appropriate, the aim was to further decrease morbidity and mortality for these critically ill patients.
Guideline adherence prior to protocol implementation was poor (24.6%) primarily due to delay in order placement and execution. Nevertheless, our mortality rate in sepsis overall was similar to published nationwide mortality of 32% at that time (16). For severe sepsis (with or without shock), our mortality was measured to be 42% which is also similar to the mortality measured by Rivers in the control group in 2001 as well as in other more recent studies (17). Importantly, implementation of the sepsis bundle was found to decrease mortality in our study group.
Treatment delay was due to failure of early recognition of sepsis, lack of coordination of care, in addition to absence of an integrated EMR and computerized physician order entry (CPOE), which further delayed order execution. While CPOE remained absent post-protocol implementation, we believe our standardized protocol helped mitigate the delays by directly alerting departments such as pharmacy and laboratory when a sepsis alert was triggered. This helped to prioritize delivery of medications and to alert physicians of critical labs, respectively. Implementation of the sepsis bundle was found to significantly decrease the time elapsed between patient presentation and performing blood cultures, administration of fluids, and antibiotic delivery.
Despite substantial improvement in antibiotic administration time, the mean time to give antibiotics was not within the recommended 60 minutes in the patients who were diagnosed with severe sepsis (with or without shock). Delays in ordering, pharmacy processing time, and medication availability, as well as lack of communication between physician, nursing, and pharmacy could reflect potential areas for further improvement.
Limitations
A potential limitation includes selection bias of patients identified for review. In the first cohort, discharge ICD-9 codes were used to obtain the population of patients with sepsis, severe sepsis and septic shock. Individual data was then examined and only those with criteria for severe sepsis or shock were included. In the second cohort, all patients who triggered a sepsis alert were analyzed. By definition, these patients met sepsis criteria with evidence of end organ damage which placed them in the category of severe sepsis with or without shock. Ultimately, both groups were required to meet the same objective criteria in order to be placed in the severe sepsis category, but the initial methodology of identifying patients was different. However, we did control for the effect of sepsis severity in our analyses to account for this difference.
The baseline severity of illness of both groups was calculated using the SOFA scoring system. Ideally, the scores should be calculated within hours of arrival to the ED. However, these scores require input of data from lab values that were often not available until much later, specifically for the phase 1 group. Consequently, we found difficulty in formulating a complete and accurate SOFA score that reflected the overall patient status on admission. In many cases in the phase 1 study, we relied primarily on laboratory data up to 48 hours into admission, which may, in turn, have artificially raised the SOFA scores, suggesting a sicker population. Due to the staggered laboratory data collection in the initial cohort, the SOFA scores should not be compared to the post protocol group, in which all patients had a complete set of laboratory values drawn at the time the sepsis alert was triggered.
Our mortality data follow-up is limited to time of discharge for both cohorts of the study. Therefore, a comparison between this data and earlier studies documenting 28-day mortality is limited.
Conclusions
Sepsis was recognized and treated sooner after protocol implementation. There was a significant decrease in mortality without change in LOS. The reduction in mortality was attributed to faster time to blood culture, antibiotic delivery, and fluid administration. An organized, streamlined approach with the incorporation of a BPA contributed to correctly identifying and initiating treatment as patients were stabilized in the ED and facilitated transfer to the ICU setting. Future studies should include and focus on 28-day mortality.
Despite the limitations of our study, we believe that a collaborative multidisciplinary approach, continuous education, and coordination of care is paramount to improve outcomes. With improved processes and continued education, the care and outcome of patients continues to improve. Despite the controversies of adherence to the SSC guidelines, our study favors that time and a protocolled approach is of the essence in the management of sepsis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the University of Miami Institutional Review Board (No. 20120298).
References
- Wang Z, Xiong Y, Schorr C, et al. Impact of sepsis bundle strategy on outcomes of patients suffering from severe sepsis and septic shock in china. J Emerg Med 2013;44:735-41. [Crossref] [PubMed]
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. [Crossref] [PubMed]
- Miller RR 3rd, Dong L, Nelson NC, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med 2013;188:77-82. [Crossref] [PubMed]
- Daniels R, Nutbeam T, McNamara G, et al. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J 2011;28:507-12. [Crossref] [PubMed]
- Levy MM, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012;12:919-24. [Crossref] [PubMed]
- Despins LA. Automated Detection of Sepsis Using Electronic Medical Record Data: A Systematic Review. J Healthc Qual 2016. [Epub ahead of print]. [Crossref] [PubMed]
- ProCESS/ARISE/ProMISe Methodology Writing Committee, Huang DT, Angus DC, et al. Harmonizing international trials of early goal-directed resuscitation for severe sepsis and septic shock: methodology of ProCESS, ARISE, and ProMISe. Intensive Care Med 2013;39:1760-75. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Centers for Disease Control and Prevention, Centers for Medicare & Medicaid Services. ICD-9-CM: International Classification of Diseases, 9th revision, clinical modification, sixth edition. Atlanta, GA: Centers for Disease Control and Prevention, 2011.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228. [Crossref] [PubMed]
- Peres Bota D, Melot C, Lopes Ferreira F, et al. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction. Intensive Care Med 2002;28:1619-24. [Crossref] [PubMed]
- Delaney AP, Peake SL, Bellomo R, et al. The Australasian Resuscitation in Sepsis Evaluation (ARISE) trial statistical analysis plan. Crit Care Resusc 2013;15:162-71. [PubMed]
- Lilly CM. The ProCESS trial--a new era of sepsis management. N Engl J Med 2014;370:1750-1. [Crossref] [PubMed]
- Wang HE, Shapiro NI, Angus DC, et al. National estimates of severe sepsis in United States emergency departments. Crit Care Med 2007;35:1928-36. [Crossref] [PubMed]
- Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014;312:90-2. [Crossref] [PubMed]
- Elixhauser A, Friedman B, Stranges E. Septicemia in U.S. hospitals, 2009. Available online: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb122.pdf
- Wang XZ, Lü CJ, Gao FQ, et al. Efficacy of goal-directed therapy in the treatment of septic shock. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2006;18:661-4. [PubMed]
Cite this article as: Ferreira TB, Diaz Y, Beg R, Wawrzyniak A, Brito Y, Burik Y, Falise J, Abbo LM, Ashley D, Lang D, Mendes E, Lupe L, Sneij W. Before and after standardizing the controversial sepsis resuscitation bundle in a large hybrid academic center. J Emerg Crit Care Med 2017;1:19.