
The use of high-flow nasal cannula in acute decompensated heart failure: ready for prime time yet?
Introduction
An ageing population, increased prevalence of chronic cardiovascular diseases and improved survival rates post-myocardial infarction has driven up the number of patients with heart failure by more than 10% in less than 5 years in the United States; from 5.7 million between 2009 and 2012, to 6.5 million between 2011 and 2014 (1). The total healthcare expenditure due to congestive heart failure is projected to increase from 24 billion dollars in 2015 to 47 billion dollars by the year 2030 (1). Acute decompensated heart failure (ADHF) constituted 2.9% of all emergency department (ED) visits in the last decade of the 20th century and has shown a steady increase across the years (2). Majority of patients with ADHF presenting to the ED requires hospitalization for further management and are 5 times more likely to need intensive care unit (ICU) admission (2). The economic and social burdens from heart failure are expected to escalate along with the silver tsunami and improved life expectancies as a result of advancement in healthcare (1).
Apart from achieving an euvolemic state, the initial management of ADHF involves relieving symptoms, and reducing preload and afterload to alleviate pulmonary congestion (3). Besides the administration of medications such as nitrates and loop diuretics, oxygen therapy is another important component in the management of ADHF, especially when complicated by hypoxemic respiratory failure. The form of oxygen therapy required (short of invasive mechanical ventilation) ranges from standard oxygen delivery devices such as face masks and non-rebreather masks, to noninvasive ventilation (NIV) through continuous positive airway pressure or bilevel positive airway pressure ventilation through sealed face masks depending on the severity of decompensation and type of respiratory failure.
Standard oxygen therapy and noninvasive positive pressure ventilation delivers oxygen that is neither humidified nor heated. Exposure to dry and cool air has been known to have deleterious effects on the respiratory mucosa, which includes impairment of mucociliary flow, induction of inflammation, and retention of mucus with resultant crusting and secondary bacterial infection (4). The therapeutic use of warm and humidified high-flow oxygen has a history dating back to the 1980s. However, the delivery of high-flow oxygen through nasal cannula has only been developed for clinical use in the past two decades (5).
The use of high-flow nasal cannula (HFNC) oxygenation has been widely adopted predominantly in the neonatal population, where avoidance of detrimental effects associated with endotracheal intubation and mechanical ventilation of preterm infants, such as lung injury and chronic lung disease is of paramount importance (6). Furthermore, HFNC has been shown to be better tolerated yet achieving similar outcomes in terms of mortality and reduced ventilator-days when compared to nasal continuous positive airway pressure ventilation in preterm infants (7). In recent years, this alternative method of oxygenation has been gaining interest in providing oxygen supplementation in adult patients with respiratory failure and various hypoxemic conditions (8). In addition to ease of administration and excellent patient tolerance, HFNC therapy has been used in outpatient settings for chronic respiratory failure (9) as well as the general ward setting (10), potentially reducing healthcare costs by averting and reducing admissions to ICUs. Despite its increasing use in treatment of acute respiratory failure from various conditions in adult patients (8), the evidence for use in ADHF is still emerging.
Physiology and mechanics of HFNC
Several physiological features of HFNC may potentially support its use in ADHF. First, the predominant mechanism of action of HFNC is through washing out of carbon dioxide in anatomical dead space by delivery of oxygen at a high flow (11), thus improving alveolar ventilation. Additionally, HFNC generates adequate flow rates to match that of the patients’ inspiratory flow in order to overcome the distensibility of the nasopharynx during inspiration and reduces work of breathing (11). As a result of the minimal difference between the delivered oxygen flow and inspiratory flow, a constant fraction of inspired oxygen (FiO2) can be maintained regardless of the patient’s respiratory minute volume (8).
Second, the use of HFNC was shown to result in lower changes in esophageal pressure swings, translating to reduced efforts in inspiration and improved lung compliance (12). The decrease in efforts of respiration is particularly important in the prevention of respiratory muscle fatigue that would necessitate endotracheal intubation and mechanical ventilation, which is associated with complications such as ventilator-associated pneumonia and longer duration of stay in the ICU (13).
Third, as oxygen delivered by HFNC is humidified and heated, the high flow is generally well tolerated by awake patients (14). Humidification and heating also facilitates mucociliary clearance of secretions, improves dyspnoea and avoids bronchoconstriction associated with inhalation of non-humidified air (11,15). The metabolic work required of the nasopharynx for gas conditioning is decreased as the gas has been pre-warmed and humidified.
Lastly, with the mouth closed, the high flow is able to maintain a positive end-expiratory pressure of up to 7.4 cmH2O (16), allowing a distending pressure to prevent atelectasis and allow lung recruitment. Although the positive airway pressure generated is relatively low compared to mechanical ventilation and non-invasive positive pressure ventilation via face mask, it is adequate enough for alveolar recruitment to improve oxygenation in mild to moderate hypoxemic respiratory failure (8). Moreover, in the presence of impaired left ventricular systolic function, the positive pressure provided by HFNC improves cardiac output and decreases afterload (17). Pulmonary vascular resistance is also decreased by improving the functional residual capacity through the positive pressure generated by the high flow in patients with pulmonary oedema (17).
Current NIV in ADHF
In patients with acute pulmonary oedema, the use of NIV with either continuous or bilevel positive airway pressure ventilation results in more rapid improvement of respiratory distress in terms of symptomatic shortness of breath through decreased work of breathing and respiratory acidosis (18). However, despite the benefits of NIV, its use is associated with disadvantages such as patient discomfort, relatively high rates of facial skin necrosis due to mechanical trauma in prolonged use, conjunctivitis induced by mask leak and the rare but serious complication of aspiration from emesis during NIV use (19). The discomfort and anxiety experienced during NIV use may result in treatment failure in patients intolerant of its application, further compounding the vicious cycle of ADHF.
When compared to HFNC, NIV use was associated with higher incidence of skin breakdown and focal erythema (20). HFNC has been shown to be better tolerated than NIV, and the use of nasal cannula as the interface for oxygen delivery instead of a face mask gives the added advantage of allowing patients to speak, resume diet and consume oral medications without any cessation in oxygenation. Furthermore, oxygenation delivered via continuous positive airway pressure had been shown to be as effective as bilevel positive airway pressure in averting the need for endotracheal intubation and carries similar mortality rates for acute pulmonary oedema (18). The lack of bilevel setting with inspiratory airway pressure in HFNC is unlikely to compromise on outcomes in normocapneic respiratory failure in patients with ADHF. Table 1 summarizes the advantages and disadvantages of both HFNC and NIV use in patients with ADHF.
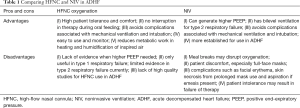
Full table
Existing evidence on HFNC use in heart failure
Positive use of HFNC has been reported for acute respiratory failure from various conditions, including pneumonia, post-extubation after cardiothoracic surgery, ICU patients with mild to moderate respiratory failure, pre-oxygenation prior to endotracheal intubation and apneic oxygenation during endotracheal intubation (14,20-24). Some of these studies included few patients with respiratory failure secondary to cardiac failure, but studies dedicated to evaluating efficacy on HFNC in ADHF are lacking.
Roca and colleagues conducted a small prospective study on 10 patients in Class III New York Heart Association heart failure to evaluate the hemodynamic and respiratory effects of HFNC therapy in such patients who were not in acute decompensation (25). The study showed that HFNC reduces the respiratory rate and causes a decrease in inspiratory collapse of the inferior vena cava (25). As the right atrium is a surrogate for right ventricular preload, and right atrial pressure is usually estimated from inferior vena cava collapsibility, the authors deduced that the decrease in inspiratory collapse indicated a reduction in right ventricular preload (25). Hence, apart from oxygenation and improving respiratory mechanics, HFNC could potentially improve cardiovascular dynamics in patients with heart failure. However, since the study was conducted in patients with stable heart failure, the generalizability of the results and efficacy of HFNC therapy in acute decompensation are subject to further evaluation. Moreover, clinically important parameters including mortality, ED recidivism and hospital readmission rates, and patient-centric outcomes such as quality of life, were not evaluated.
HFNC therapy has also shown some encouraging results in treating patients who developed hypoxemia following NIV use in the ED. A case series of five patients in ADHF who were treated with HFNC after NIV use in the ED demonstrated improved degree of dyspnoea with high degree of comfort (26). All five patients experienced moderate (20%) to severe (80%) dyspnoea, which improved significantly with a decrease in work of breathing and tachypnea, and significant improvement in arterial oxygen saturation from 85% to 99% after 24 hours of HFNC administration (26). Although two patients experienced some tracheal discomfort, it was well-tolerated after the initial adaptation period and did not result in therapy withdrawal (26).
Only one randomized controlled trial has been conducted and published thus far on use of HFNC in the treatment of cardiogenic pulmonary edema presenting to the ED (27). In this study conducted by Makdee and colleagues in a single center ED in Thailand, adult patients with a diagnosis of cardiogenic pulmonary oedema were randomized into two arms of intervention: conventional oxygen therapy with nasal cannula or non-rebreather mask versus HFNC oxygenation at a flow rate of 35 to 60 L/min. The primary outcome of respiratory rate per minute was evaluated at 60 minutes after initiation of assigned therapy. Although the study showed statistically significant reduction in respiratory rate at 15-, 30- and 60-minute post-initiation of HFNC therapy compared to conventional oxygen therapy, the differences were too small to translate to clinical significance (mean differences ranged from 1.8 to 3.3 breaths/min) (27). In addition, the study excluded patients with SpO2 of less than 90% and respiratory rate of 35 breaths per minute or more, making the results of the study implausible to be extrapolated to heart failure patients with severe hypoxemia and respiratory distress. The surrogate outcome of decrease in respiratory rate was not accompanied by more clinically important outcomes such as intubation rates and mortality, which should be the crux of evaluation in future studies. Table 2 provides a summary on studies conducted using HFNC in patients with heart failure.
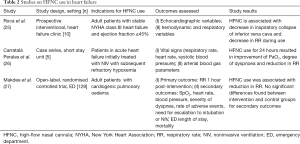
Full table
Conclusions
HFNC may be a useful form of therapy in the treatment of selected patients with ADHF due to its ease of application and excellent patient tolerance in addition to its beneficial physiological properties. The paucity of dedicated studies evaluating clinically important outcomes provides opportunities for larger well-designed studies in the future to assess its efficacy in ADHF.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146-e603. [Crossref] [PubMed]
- Hugli O, Braun JE, Kim S, et al. United States emergency department visits for acute decompensated heart failure, 1992 to 2001. Am J Cardiol 2005;96:1537-42. [Crossref] [PubMed]
- Onwuanyi A, Taylor M. Acute decompensated heart failure: pathophysiology and treatment. Am J Cardiol 2007;99:25D-30D. [Crossref] [PubMed]
- Déry R, Pelletier J, Jacques A, et al. Humidity in anaesthesiology. 3. Heat and moisture patterns in the respiratory tract during anaesthesia with the semi-closed system. Can Anaesth Soc J 1967;14:287-98. [Crossref] [PubMed]
- Waugh J. High flow oxygen delivery. Trends of non-invasive respiratory support: continuum of care. Clin Found 2009;1-12.
- Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev 1998;53:81-94. [Crossref] [PubMed]
- Shoemaker MT, Pierce MR, Yoder BA, et al. High flow nasal cannula versus nasal CPAP for neonatal respiratory disease: a retrospective study. J Perinatol 2007;27:85-91. [Crossref] [PubMed]
- Nishimura M. High-flow nasal cannula oxygen therapy in adults. J Intensive Care 2015;3:15. [Crossref] [PubMed]
- Bräunlich J, Seyfarth H-J, Wirtz H. Nasal High-flow versus non-invasive ventilation in stable hypercapnic COPD: a preliminary report. Multidiscip Respir Med 2015;10:27. [Crossref] [PubMed]
- Pirret AM, Takerei SF, Matheson CL, et al. Nasal high flow oxygen therapy in the ward setting: a prospective observational study. Available online: http://dx.doi.org/ [Crossref]
- Dysart K, Miller TL, Wolfson MR, et al. Research in high flow therapy: mechanisms of action. Respir Med 2009;103:1400-5. [Crossref] [PubMed]
- Mauri T, Turrini C, Eronia N, et al. Physiologic Effects of High-Flow Nasal Cannula in Acute Hypoxemic Respiratory Failure. Am J Respir Crit Care Med 2017;195:1207-1215. [Crossref] [PubMed]
- Antonelli M, Conti G, Rocco M, et al. A comparison of non-invasive positive pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med 1998;339:429-35. [Crossref] [PubMed]
- Parke RL, McGuinness SP, Eccleston ML. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care 2011;56:265-70. [Crossref] [PubMed]
- Rea H, McAuley S, Jayaram L, et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med 2010;104:525-33. [Crossref] [PubMed]
- Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care 2007;20:126-31. [Crossref] [PubMed]
- Inata Y, Takeuchi M. Complex effects of high-flow nasal cannula therapy on hemodynamics in the pediatric patient after cardiac surgery. J Intensive Care 2017;5.-30 [PubMed]
- Gray A, Goodacre S, Newby DE, et al. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med 2008;359:142-51. [Crossref] [PubMed]
- Hotchkiss JR, Marini JJ. Noninvasive ventilation: an emerging supportive technique for the emergency department. Ann Emerg Med 1998;32:470-9. [Crossref] [PubMed]
- Stéphan F, Barrucand B, Petit P, et al. High-Flow Nasal Oxygen vs Noninvasive Positive Airway Pressure in Hypoxemic Patients After Cardiothoracic Surgery: A Randomized Clinical Trial. JAMA 2015;313:2331-9. [Crossref] [PubMed]
- Vourc'h M, Asfar P, Volteau C, et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med 2015;41:1538-48. [Crossref] [PubMed]
- Sztrymf B, Messika J, Mayot T, et al. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: a prospective observational study. J Crit Care 2012;27:324.e9-13. [Crossref] [PubMed]
- Miguel-Montanes R, Hajage D, Messika J, et al. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med 2015;43:574-83. [Crossref] [PubMed]
- Hernández G, Vaquero C, Colinas L, et al. Effect of Postextubation High-Flow Nasal Cannula vs Noninvasive Ventilation on Reintubation and Postextubation Respiratory Failure in High-Risk Patients: A Randomized Clinical Trial. JAMA 2016;316:1565-1574. [Crossref] [PubMed]
- Roca O, Pérez-Terán P, Masclans JR, et al. Patients with New York Heart Association class III heart failure may benefit with high flow nasal cannula supportive therapy: high flow nasal cannula in heart failure. J Crit Care 2013;28:741-6. [Crossref] [PubMed]
- Carratalá Perales JM, Llorens P, Brouzet B, et al. High-Flow therapy via nasal cannula in acute heart failure. Rev Esp Cardiol 2011;64:723-5. [PubMed]
- Makdee O, Monsomboon A, Surabenjawong U, et al. High-Flow Nasal Cannula Versus Conventional Oxygen Therapy in Emergency Department Patients With Cardiogenic Pulmonary Edema: A Randomized Controlled Trial. Ann Emerg Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Chua MT, Kuan WS. The use of high-flow nasal cannula in acute decompensated heart failure: ready for prime time yet? J Emerg Crit Care Med 2017;1:22.


