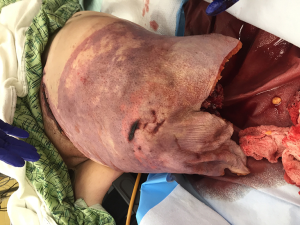
Lower extremity traumatic amputations—brief review of reconstruction, wound and soft tissue management, alternatives to conventional practice
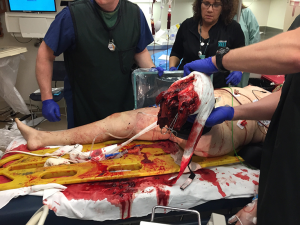
Treatment of traumatic amputees will always focus on initial stabilization and bleeding control with preservation of life, limb eyesight. After initial resuscitation, the residual limb is treated as a secondary matter, resected proximally and wound closed as soon as it is expeditious. All too frequently, completion of an amputation is relegated to junior members of the surgical staff, with re-amputation and shortening as the primary goal, ensuring a rapid wound closure. Additionally, amputations performed above the zone of injury, particularly for transfemoral amputees (“high -femoral”), would leave them with fewer prosthetic options, which also necessitates an approach focusing on more long-term outcomes (1). Recent advancements in trauma care during the Afghanistan and Iraq campaigns have opened discussion regarding the application of Osteomyoplastic amputation as a primary procedure rather than a secondary reconstructive option (2-5). We have taken a more in-depth approach to traumatic amputations, which would allow, over a long-term, more of a functional return, utilizing an approach of expectant wound care and delayed reconstruction based on the patient’s clinical condition and presentation, with osteomyoplastic amputation as a primary procedure (Figure 1).
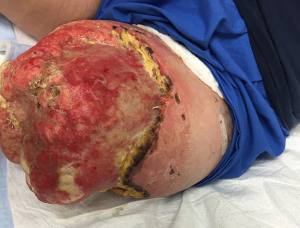
Osteomyoplastic reconstruction is a technique that was devised after World War I to accommodate a shortage of prosthetics by creating a distal, end-weight bearing residual extremity, which the patients could use to support their own body weight (6,7). Despite the plethora of prosthetic options and improved knowledge of fitting, this principle could still be applied today. As a reconstructive option, it allows greater flexibility to the patient, for prosthetic wear, and functionality, and enhances a return to a more functional pre-injury state (7,8) (Figure 2).
Brief history
Osteomyoplastic amputation and reconstruction was developed and perfected by Dr. Janos V. Ertl in Budapest, Hungary, as a way of providing a functional residual limb for tens of thousands of traumatic amputees from trench warfare in World War I (1,6,7). With a lack of funding or usable prosthetics for so many wounded veterans who could otherwise be gainfully employed, he devised a method using native periosteum to create a synostosis between the distal tibia and fibula, myoplasty which would allow more functional engagement of the lower limb. These patients ultimately could stand on most or all their body weight on the residual limb and allow them to continue working with any type of prosthetic that could be made either by themselves or manufactured. During the early 20th century most work was still performed by hand and required ambulation or standing at one’s workplace.
While providing distal end-weight bearing to below-knee amputees (BKA), trans-femoral amputees (above-knee amputees, AKA) also benefited by the creation of an osteoperiosteal end-cap of the femur (avoiding crown sequestration) and muscle balancing of the adductors to abductor’s, and rectus femoral to the hamstrings. This also allows distal end weightbearing prosthetic as well as providing a bi-directional myoplasty, giving greater control of the prosthetic limb (1,6,7).
Effects of conventional amputation (8-12)
- Majority of musculature allowed to retract; which undergo fibro-fatty atrophy leading to poor “volume” of a residual extremity; lack of a muscle “pump” initiates venous stasis; slower speed of contraction; eventual poor distal coverage due to a lack of soft tissue. stabilization > unstable osseous structures > bell clapping of femur (AKA), fibular motion (BKA);
- Increased metabolic activity and work-of ambulating due to “inactive residual extremity”;
- AKA have the femur transected above the epicondyles and muscles are shortened to fit vs. myoplastic apposition. Regional circulation disturbed both to soft tissue and to bone, secondary to venous stasis, abnormal vessel formation, high risk of arteriovenous malformation (AVM), dilated, tortuous vessels (13).
Osteomyoplastic reconstruction provides an amputee with a stable platform, allowing them to ambulate with any type of prosthetic or engage in any reasonable activity they are physically capable of.
Utilizes a three-phase approach
- “Bone-bridge”: using native periosteum as a living, autologous graft, which ultimately forms an osteal bridge, fusing the tibia and fibula together, or for primary amputees a section of viable bone is used as an interposition graft, and for trans-femoral amputees an “end-cap”;
- Myoplasty: muscles are re-opposed to each other using anatomical attachments (i.e., antero-lateral to posterior which also creates a larger soft tissue envelope, further increasing surface area; for trans-femoral balances anterior-posterior and medial-lateral groups;
- Placement of incisions away from potential load bearing areas and/or across a broader area, and meticulous, multi-layer, plastic wound closure.
Primary amputation with immediate osteomyoplastic reconstruction transtibial
Existing tissue planes are identified, and myofascial flaps are examined for distal soft-tissue coverage. Muscles are then mobilized as a contiguous group, or anatomic unit. Neurovascular structures are now isolated, released from scar tissue and resected separately, to allow retraction into the proximal muscle group. Often, during times of crisis, they were ligated en-masse and are difficult to separate, so careful proximal dissection should be done. Identification and isolation of all nerves, specifically: saphenous, sural, lateral sural, deep and superficial peroneal, posterior Tibial branches deep and superficial.
Osteoperiosteal graft is harvested using a 1-inch chisel and double-tap hammer blows, a periosteal-osteo flap is harvested moving distal to proximal “up the tibia”; that is cortical fragments are left attached to the medial periosteum of the tibia, the procedure is the same for the fibula. Bone grafts are best harvested from resected fibula, for short residual limbs, the fibula can be split, or commercial bone (dowels) or banked bone can be utilized, or autologous bone can be morselized. The periosteal graft is wrapped around the bone graft and secured with 0-Vycril and fashioned into a tube. Previously mobilized muscles are brought distally, as groups, antero-lateral and gastrocnemius, and are sutured to each other in a pants-over-the-vest fashion (myoplasty vs. myodesis) (1,6).
Primary amputation with immediate osteomyoplastic reconstruction transfemoral
As indicated above, tissue planes are identified, and flaps are mobilized based on medial-lateral, and anterior posterior muscle insertions. Resection of nerves, and retraction out of the healing-zone typically includes sciatic, obturator, saphenous and superficial nerves (if identified).
The medullary canal for transfemoral amputees is closed by forming an “end-cap” of osteo-periosteal flap(s), rather than a “bridge”, this will avoid crown sequestration. For transfemoral amputees, key elements are muscle balancing and correcting “lateralization” of the femur (14). This is accomplished by first suturing the medial (adductors) preferentially attach past the lateral (abductors). If not restored, adductor movement allows the femur to lateralize creating an inefficient gait pattern, increasing oxygen demand and creating greater cardiac stress in patients with cardiopulmonary disease. If possible, maintaining the adductor magnus and gracilis muscles will help to restore the adductor moment (6,7,10,11). Transfemoral myoplasty is completed by suturing the quadriceps to the hamstrings. This stabilizes the entire soft tissue envelope and provides distal coverage for end-bearing of the residual limb.
Wound closure (10,11)
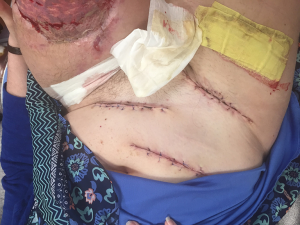
Meticulous skin closure is then performed, removing dog-ears and redundant skin, with an end-goal of providing a cylindrical limb for prosthetic application. Closed suction drains are regularly placed and left at least until the first dressing change, or longer based on clinical exam. Multi-layered closure is done principally with Vicryl to relieve tension on the epidermis, with final closure either staples or sutures, both are equally efficacious. For patients with skin loss, full-thickness skin grafts have the distinct advantage of allowing dermal regeneration and are better able to withstand constant rubbing/friction from total contact, end-weight bearing sockets, such as used with Osteomyoplastic amputees. These are the preferred choice, in addition to split-thickness grafts. Donor sites are usually available on the inguinal and abdominal regions (Figure 3).
Dressings
Dermal contact layer is typically non-adherent at the incision line them layered gauze applied, all amputees benefit from compression, and there are several methods to effect edema control.
Semi-rigid dressing (13,15)
Most common and available is the Unna boot: zinc-oxide/calamine/gelatin/glycerin, which are applied in the OR and left in place, undisturbed for 5–7 days. Common problems are itching leading to scratching when dry, no access to concerning wound(s), and no protection from trauma (all amputees fall).
Short-splint
Typically, a plaster or fiberglass cast, either as a long section from a roll, or as layered plaster, secured to one plane/surface (a.k.a. posterior splint/mold), immediately applied prior to anesthesia reversal. Advantages include protects wound from trauma, compression (with elastic bandage), inexpensive, and readily available. Disadvantages include blister formation, may be heavy/bulky, and draining wounds can be problematic, weakening the plaster.
Rigid cast/splint (15-17)
Made from plaster or fiberglass and applied circumferentially (vs. single plane); typically, are heavy and cumbersome for patients (Figure 4).
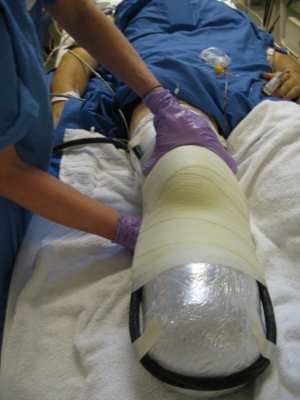
Removable rigid dressing (RRD) (15-17)
This is our method of choice, and is manufactured/fabricated by a prosthetist, has the same characteristics as the rigid dressing but lighter materials (polypropylene). Uses layers of socks and a silicone gel-liner to provide the necessary compression vs. a shrinker. Using plastic allows for easier cleaning and as well as rigid stability, and circumferential protection. Can be pre-made or custom molded to the residual limb using a specific wrap technique. Disadvantages include the fact that it does not work when the patient removes it off by themselves (especially when they have not been trained); it is not designed for pressure bearing ambulation; initial post-op edema can be problematic, therefore over-size should be projected, as well as adequate room on initial design for dressing. Having two separate halves (“bivalve”) will avoid distal compression from pivoting on a “hinge”, and causing constriction, multiple adjustable straps, and accounting for dressings and edema, avoids over-compressing the limb (better slightly too big for the than too small, and socks and gauze can fill the gaps) (Figure 5) (18).
Timing of dressing changes and/or frequency
While there is no established guideline, wound inspection is more often based on clinical concern, vs. staff convenience; typical considerations for timing: ongoing bleeding or oozing, soilage, odor, pain out of proportion to what is expected, patient voices discomfort, or objective clinical concern temperature >101.5 °F/elevated white cell count. Our preference is 2–3 days post-operatively to moderated edema and allow for careful compression and hemostasis.
Skin/soft-tissue defects
Often, with poly-trauma patients there remains soft-tissue/skin defects, which may or may-not be significant in size or location. With any of these patients, a true multi-disciplinary approach is required, and early consultation is more appropriate than a request made to “plastic surgery” at the “point of need”. We prefer the former, as it allows for long-term solutions vs. a rapid skin graft to cover an open wound. Closing the wound to expedite prosthetic fit, or discharge, in an otherwise healthy patient may be at the expense of chronically poor fit over time (19). Split thickness grafted skin does not maintain the ability to regenerate as well as full-thickness skin, nor does it perspire. Friction with the socket/liners becomes an issue therefore, leading to ulceration and pain, which then decreases their prosthetic wear. For these reasons, utilizing a staged approach to wound healing, while appearing longer in the acute state, allows for more durable options in the recovery/rehabilitation phase. Nonetheless there are certain wound-management principles which invariably should be followed for these patients (Figure 6):
- Surgical debridement of all necrotic, purulent, and questionably viable tissue is mandated (19);
- Appropriate antibiotics selection and duration (evidence based vs. dogmatically choosing 10–14 days);
- Aggressive medical management of glycemia, and sepsis;
- Other adjuncts if available (e.g., hyperbaric oxygen therapy, elevation, graded compression);
- Daily wound evaluation by trauma team;
- Avoiding single-modality wound treatments’ ad infinitum, i.e., wet/dry, or negative pressure wound therapy, without re-evaluation;
- Debriding treatments can be alternated, for instance, ¼–½ strength Dakin’s solution (sodium hypochlorite) for 1 or 2 weeks, advancing to moist/dry saline (BID or TID); will cause an inflammatory rind/peel that can wall-off open areas, and treat gross contamination without prolonged irritation of healthy peri-wound tissue to the desiccating effect of bleach compounds;
- ¼ strength acetic acid is also indicated for Pseudomonas overgrowth, as mostly this is a contaminant in the bioburden of the wound vs. true invasive infection (usually 1 or 2 weeks only).
Once granulation appears and there is no more necrotic debris, then shifting focus to maintaining a clean bed for closure can then incorporate more chronic therapeutic options. That is Negative pressure wound therapy, or calcium alginate, or even dermal substitutes can be utilized with success (nota bene- depending on payor allowances, reimbursement may be denied, therefore pre-approval should be done before applying) (Figure 7).
Summary
The technique for osteomyoplastic amputation as compared to conventional (Burgess) amputation (2-4,8,9,13) as described above, in addition to our wound care strategies, allow us to manage these complicated patients and still provide a long-term, functional outcome. There is a large enough body of evidence from military medical colleagues during the Afghanistan and Iraq campaigns, that strongly suggests that clean wounds in stable patients can be left to granulate, and aggressive debridement above the zone of injury is not always indicated (19), therefore expedient closure is not necessary. With an aggressive approach to wound care even a “bad” or mangled limb can have potential for salvage and decreasing functional loss. Adopting an approach of expectant wound care with delayed reconstruction allows a longer term, more functional outcome for the amputee versus immediate prosthetic fit which may or may not require subsequent revisions.
Variation in surgical skill level abounds in every institution, yet most Osteomyoplastic principles, when broken into components, are taught to every surgical specialty, and should not preclude a thoughtful, pro-active approach to the traumatic amputee. Complications with trauma patients happen, whether surgical or medical, and should be managed no differently in amputees.
If they have the capacity at the time of injury, most patients would not want an amputation, yet once the injury has occurred the damage may be done. While salvage of length is the goal with this article there are those patients who benefit from earlier primary amputation vs. protracted surgeries for limb-salvage of a non-functional extremity. Psychological issues surrounding amputation still abound, are too numerous, and important to give a hollow overview in the constraints of this article, nonetheless offering Osteomyoplastic amputation is an opportunity to give back a “choice” to an acutely injured patient.
Outcomes for osteomyoplastic amputation have been studied over recent (2,4,5) as well as previous conflicts (3,8,9,14), with results documented, and despite patient and provider acceptance, there remains reticence to employ it in the trauma toolbox. While most patients in the civilian world readily accept this procedure, and have good outcomes (20), debate continues as to what constitutes irrefutable data. Several studies that have focused on retrospective analysis of patient satisfaction, have a low “N”, and do not account for experience of the prosthetic provider, what constitutes a “good fit”, as well as the whether the surgical concepts of Osteo myoplasty, as described, were truly followed (2,4,5). Further counter-arguments anecdotally include, higher complication rate, delayed prosthetic fit, increased surgical time, etc. I would opine that, as with any procedure, there is a learning curve, and time in the Operating Room is well spent when it results in a durable, functional residual limb, additionally, complications occur with any procedure, and a thorough knowledge of their management in implied in our training. Ongoing studies such as “TAOS” will, hopefully, account for the multiple of variables and further clarify osteomyoplastic surgery as an acceptable option in trauma. Borrowing from the military experience and utilizing the “ladder of reconstruction”, simple techniques may be adequate before advancing to more complex options, and osteomyoplastic amputation can be both simple and complex based on skill, dedication, and a knowledge of the “scalpel as an instrument of healing” (2,20).
Acknowledgements
The author would like to thank Tim Darling (CPO), Lissa Adams (MPT, CP, RD) for their invaluable assistance in dressings and wound management; and William Ertl (MD), John W. Ertl (MD) for their dedication in passing on osteomyoplastic reconstruction the surgical world.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Murdoch G. Levels of amputation and limiting factors. Ann R Coll Surg Engl 1967;40:204-16. [PubMed]
- Keeling JJ, Shawen SB, Forsberg JA, et al. Comparison of functional outcomes following bridge synostosis with non-bone-bridging transtibial combat-related amputations. J Bone Joint Surg Am 2013;95:888-93. [Crossref] [PubMed]
- Dougherty PJ. Transtibial amputees from the Vietnam War. Twenty-eight-year follow-up. J Bone Joint Surg Am 2001;83-A:383-9. [Crossref] [PubMed]
- Plucknette BF, Krueger CA, Rivera JC, et al. Combat-related bridge synostosis versus traditional transtibial amputation: comparison of military-specific outcomes. Strategies Trauma Limb Reconstr 2016;11:5-11. [Crossref] [PubMed]
- Bosse MJ, Morshed S, Reider L, et al. Transtibial Amputation Outcomes Study (TAOS): Comparing Transtibial Amputation With and Without a Tibiofibular Synostosis (Ertl) Procedure. J Orthop Trauma 2017;31 Suppl 1:S63-9. [Crossref] [PubMed]
- von Ertl J. Regeneration, ihre Anwendung in der Chirurgie: mit einem Anhang Operationslehre. Barth, 1939.
- Ertl J. Uber Amputationsstumpfe. Chirurg 1949;20:218.
- Loon HE. Biological and biomechanical principles in amputation surgery. In: International Prosthetics Course, Second Proceedings. Committee on Prosthesis, Braces, and Technical Aids; Copenhagen, 1960:41-58.
- Loon HE. Below-knee amputation surgery. Artificial Limbs National Academy of Sciences-National Research Council 1962;6:86.
- Dederich R. Stump correction by muscle-plastic procedure. In: International Prosthetics Course, Second Proceedings. Committee on Prosthesis, Braces, and Technical Aids; Copenhagen, 1960:59-61.
- Dederich R. Plastic treatment of the muscles and bone in amputation surgery. A method designed to produce physiological conditions in the stump. J Bone and Joint Surg 1963;45B:8.
- Hansen-Leth C, Karle A. Intracardial arteriographic study on vascular changes in amputated rabbits. Acta Orthop Scand 1978;49:457-63. [Crossref] [PubMed]
- Burgess EM, Romano RL, Zettl JH. The management of lowerextremity amputations: surgery, immediate postsurgical prosthetic fitting rehabilitation. Washington, DC: US Government Printing Office, 1969.
- Gottschalk F. Transfemoral amputation. Biomechanics and surgery. Clin Orthop Relat Res 1999.15-22. [Crossref] [PubMed]
- Choudhury SR, Reiber GE, Pecoraro JA, et al. Postoperative management of transtibial amputations in VA hospitals. J Rehabil Res Dev 2001;38:293-8. [PubMed]
- Mueller MJ. Comparison of removable rigid dressings and elastic bandages in preprosthetic management of patients with below-knee amputations. Phys Ther 1982;62:1438-41. [Crossref] [PubMed]
- Ladenheim E, Oberti-Smith K, Tablada G. Results of managing transtibial amputations with a prefabricated polyethylene rigid removable dressing J Prosthet Orthot 2007;19:2-4. [Crossref]
- Hanger Clinic, Kiwi Course, Internal publication, 2007. Available online: http://hangerclinic.com/
- Nessen SC, Lounsbury DE, Hetz SP. editors. War surgery in Afghanistan and Iraq: a series of cases, 2003-2007. Falls Church, Va.: Office of the Surgeon General, United States Army, 2008.
- Ertl JW, Ertl JP, Ertl WJ, et al. The Ertl Osteomyoplastic Transtibial Amputation Reconstruction: Description of Technique and Long Term Results. The Annual Meeting of the American Academy of Orthopaedic Surgeons; 1997 Feb 13-17; San Francisco, CA.
Cite this article as: Ertl CW. Lower extremity traumatic amputations—brief review of reconstruction, wound and soft tissue management, alternatives to conventional practice. J Emerg Crit Care Med 2018;2:35.