
Novel use of interventional radiology in trauma
Introduction
Trauma is the third overall leading cause of mortality across all ages (1). It remains the leading cause of death among Americans 1 to 44 years old causing 193,000 deaths annually. Exsanguination is the most common cause constituting up to one-third of deaths. Currently, there is an emphasis on non-surgical management of hemodynamically stable patients who have suffered traumatic injuries. With recent advances in endovascular techniques, there is an increasing role for it in the management of traumatic hemorrhage (2). Prospective randomized controlled trials are difficult to perform in the setting of trauma for several reasons. The most common reasons include the variability in the type and extent of injuries, clinical status of presentation, pre-existing conditions and comorbidities and hyper acute treatment scenarios. Consequently, there is lack of level 1 evidence for the management of these patients (2,3).
Due to the advances in the field of radiology in its entirety, we see a greater role interventional radiology (IR) specifically plays in the management of patients who have suffered traumatic injuries. Diagnosis and characterization of solid organ and major vascular injuries are primarily done by multi-detector computed tomography (CT), limiting the role of angiography for therapeutic and problem-solving scenarios. As the diagnosis is made, the interventionalist can step in and utilize advanced endovascular techniques to finely carry out distal embolization with micro-catheters and newer embolic agents or offer stent grafts of various sizes (2).
For efficient management of trauma patients, a standardized institutional workflow algorithm for effective and time sensitive triaging of them is recommended using a multidisciplinary team comprised of an orthopedic surgeon, anesthesiologist, radiologist, interventional radiologist, neuro-interventional radiologist, trauma surgeons, critical care physicians, and emergency room physicians, all working and coordinating together. The on-call vascular interventional radiology (VIR) team should be composed of an interventional radiologist, an assistant (fellow, resident scrub tech), a nurse (with critical care experience), and an IR technologist.
Early triaging is critical in deciding management options, which range from observation, IR (with or without hybrid CT technology), or open surgery. A combination suite well equipped with a fluoroscopic unit and a dual source CT scanner with a conventional sliding table is ideal. It provides quick imaging, accurate diagnosis, early procedural triaging, and potentially shortens the time from diagnosis to intervention. In this scenario, trauma surgeons could have easy access to operate without having to move the patient from the interventional table to the operating room.
Resuscitation of hemodynamically unstable patients takes precedence over the procedure. Since multiple injuries with trauma is a rule rather than an exception, continuous assessment and triaging based on the severity of the injury and vital organs involved is essential. It is useful in determining the appropriate next step in management. Deciding between surgeries, IR or a combined procedure early on is imperative and can potentially change outcomes for these patients. For example, in a patient with traumatic brain injury and pelvic trauma, after initial hemodynamic resuscitation, a decompressive craniectomy holds a higher priority compared to pelvic angiogram with embolization. Similarly, with an unstable pelvic fracture external fixation and pre-peritoneal packing take precedence over an angiogram and embolization.
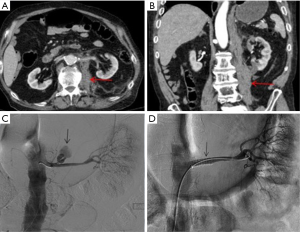
A hybrid room can accommodate both IR and surgical intervention, which can significantly save time when it is crucial for patients (Figure 1) (4).
A hybrid room equipped with a CT scanner in the angiography suite can provide critical real time information in detecting small vessel bleeds. The inherent three-dimensional properties of a CT scanner can more accurately identify a bleeding vessel when used in conjunction with digital subtraction angiography (DSA) (Figure 2). This is important, since a motion filled background limits visualization of a bleeding vessel.
Additionally, angiographic C-arms are equipped with flat panel detectors (FD-CT), which allow for rotational cone beam acquisition of X-ray, makes it possible to acquire CT like images in the angiography suite, providing useful anatomical information and accurate depiction of active bleeds. It is helpful in visualizing irregular branches in areas of concern as well and to monitor the effectiveness of the embolization in the angio-suite (5,6).
These images are then reconstructed into a 3D image work station using a software algorithm. The newer machines have a higher speed of acquisition, lower radiation dose, reduced need for contrast and reduction in procedure time (7). The commercial names include Innova CT HD (GE Healthcare, Chalfont St. Giles, UK), DynaCT (Siemens AG, Healthcare Sector, Forchheim, Germany), INFX-8000C + CT (Toshiba Medical Systems, Tochigi, Japan), XPerCT (Philips Healthcare, Andover, MA, USA) and Safire 3D-C (Shimadzu, Kyoto, Japan) (8). 3D rotational DSA if used on initial workup, has been shown to reduce the incidence of false negatives in identifying the bleeding vessel (9).
The role of information technology
Advances in technology have improved workflow and triaging. Repeat CT in trauma patients transferred between hospitals increases radiation exposure and cost. More importantly, it increases the critical time interval between the traumatic event and potentially lifesaving procedures. Cloud based, image sharing technology (such as lifeIMAGE) is increasingly being used to share the images across the trauma systems, making the images available to the referred hospital within seconds. In addition to eliminating/reducing the need for repeat CT, quick access to the images gives the receiving hospital ample time for triaging and planning potential treatment options. Also, it reduces radiation exposure and cost associated with repeat CT (10).
To decrease the response and turnaround time, hospitals provide home based picture archiving and communications system (PACS) for on-call radiologists and interventional radiologists to review images and formulate a plan of action before reaching the hospital.
In addition to telephones and pager systems, newer devices have seen increasing role in hospital communications. Voice activated hands-free communication devices (Vocera) have resulted in improved turn around and response times (11).
The utility of earlier methods of communication, like a pager, have become limited due to extensive use of smart phones. However, most hospitals continue using pagers due to HIPAA compliance issues associated with mobile text messaging. Still, approximately 58% of residents and 15–19% of physicians have self-reported violating HIPAA by sharing PHI via text messages (SMS). Physicians in surgical specialties compared to the non-surgical specialties are more likely to violate HIPAA via SMS use (35% vs. 17%). The most common barrier to complying with HIPAA was the inconvenience (58%) due to the traditional HIPAA compliant modes of communication, like pagers (12). The role of HIPAA compliant encrypted messaging services, such as TigerText, have solved that problem. In addition to the ease of communication and being HIPAA compliant, these messenger services have advanced tools like the photo and video sharing, which enhances the quality of communication. Patients’ radiology images or gross pictures can be shared. Although the image quality is not state of the art and can only be as good as the device on which the software is installed, it provides more information in less time.
Angiography and embolization (AE)
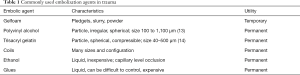
AE is the most commonly utilized IR procedure in the setting of acute trauma. Embolization is an intentional occlusion of a vessel to arrest blood flow by the introduction of embolic material. The primary goal of embolization is to control the hemorrhage at its source intravascularly. The secondary goal is to prevent embolic material traveling to non-targeted organ tissue, thereby preventing organ dysfunction and associated sequelae. The choice of embolic agent varies based on the targeted bleeding organ/vessel (2). The timing in the use of embolic agents is also critical. Most commonly used embolic agents in embolotherapy are described in Table 1 (15).
Full table
Most embolic agents require a functioning coagulation cascade to work efficiently. Hence, embolization should be performed as soon as possible before the onset of coagulopathy. Coagulopathy is more common in patients with hypothermia and with major hemorrhage requiring multiple blood transfusions (13).
Some embolic agents, like N-butyl cyanoacrylate (NBCA) (Trufill, Codman, Raynham, MA, USA) and ethylene vinyl alcohol (EVOH) copolymer (Onyx, Covidien, Plymouth, MN, USA) are useful even in the setting of coagulopathy. The cost, availability and a steep learning curve preclude its widespread use.
NBCA readily polymerizes in contact with water and has good tensile strength. It is mixed with plasticizers and stabilizers which increase its flexibility and reduces toxicity (14). NBCA is primarily a tissue adhesive used for surgical wounds and traumatic laceration closure. Endovascular use of NBCA has various indications including: venous malformations, pseudoaneurysms, varicocele, ectopic varices, preoperative embolization of meningioma, selective embolization of bone lesions and large vessel embolization to control hemorrhage (16-20).
EVOH is a new liquid embolic copolymer predominantly used in neuro-interventional procedures and chronic aortic endoleaks. However, physical properties like viscous nature, slow polymerization, ability to conform to vessel shape and high fluoroscopic visibility have led to its increasing use for embolization in peripheral vessels. EVOH is more commonly utilized in the treatment of hemoptysis and lower GI bleed (21).
Detachable coils have revolutionized the field of neuro-intervention by providing safe and consistent endovascular treatment of aneurysms with a relative decline of clipping procedures (22). After the initial development of commercially available helical detachable coils in 1991, many different kinds of detachable coils with the various mechanism of the detachment were developed (23). The most popular types are three-dimensional coils (framing coils), finishing coils, Interlock Fibered IDC Occlusion System (Boston Scientific, Natick, MA, USA) and HydroCoils.
The helical coils’ two-dimensional morphology does not consistently allow good neck coverage during embolization of larger aneursms. Later on, the helical design was modified (24) and a new prototype detachable coil was developed by Guglielmi and was called Guglielmi Detachable Coils (GDC) (Target Therapeutics, Fremont, CA, USA). It uses a small electrical current to release a micro-soldered joint (25), and thus release the coil from the delivery system.
Three-dimensional coils have an intrinsic spherical shape that it acquires and maintains after being deployed into the aneurysmal sac. They were later referred to as framing coils. Although not statistically proven, it is intuitive that use of these framing coils resulted in fewer attempts at repositioning, more reliable retention within an aneurysm after detachment, and reduced procedure and fluoroscopy time. Subsequently, newer types of framing coils were introduced like, Microsphere [Micrus (now Codman), Raynham, MA, USA], Compass and Cosmos (MicroVention, Tustin, CA, USA) and Axium 3D [ev3 (now Covidien), Irvine, CA, USA]. These are relatively robust coils with a tendency towards three-dimensional deployment (24).
Finishing coils were introduced to fill up the irregular residual spaces in the helical and framing coil mass which is too small to be filled in by the smallest available helical coils. Finishing coils are softer (0.010 inches) with more filamentous core and less tightly wound secondary winds, making it suitable for fitting into small irregular residual spaces (24).
The Interlock Fibered IDC Occlusion System (Boston Scientific) was introduced to clinical practice in 2006 (15). Interlock coils have a high memory performance and a tendency to retain its original shape. They are bendable and track easily through curves. The coil and pusher have interlocking mechanical arms that keep them locked until the interlocking arms are in the delivery catheter. It allows for a controlled release of the coil with an ability to retract the coil for repositioning or retraction before final placement (15).
In addition to embolic agents and detachable coils, microvascular plugs have improved outcomes. Microvascular plug (MVP, UNO; Reverse Medical Corp, Irvine, CA, USA) is designed for occlusion of small vessels accessible only by micro-catheters. They are used for management of an aneurysm and acute or imminent hemorrhage. When appropriate size microvascular plugs are used, it causes an immediate arrest of bleeding with persistent hemostasis, proven on follow up studies (26).
In non-selective angiography, an extravasation rate of 1 cc/min is required to detect hemorrhage if the catheter is located more than 10 cm from the injured segment. In super-selective angiography, with the catheter positioned within 1 to 2 cm from the injury site, the threshold for detection of active extravasation is 0.1 cc/min (27).
Potential complications associated with AE include femoral artery dissection, femoral arteriovenous fistula (AVF) formation and coil migration (28).
Thoracoabdominal trauma
The availability, speed and spatial resolution of multi-detector CT scanners have improved diagnostic efficacy in traumatic injury, with whole body scan acquisitions performed within minutes. The site and extent of the injury are identified faster with high accuracy. Hence, the evaluation and management protocols for trauma have changed. The role of CT scan in the evaluation of trauma patients has been increasing relative to focused assessment with sonography for trauma (FAST), diagnostic paracentesis and conventional angiography. FAST continues to be a useful radiation free tool for bedside evaluation of unstable patients. Diagnostic accuracy of contrast-enhanced ultrasound is comparable to CT scan for identification of solid organ injury (29). Patients responding well to initial resuscitation are subsequently evaluated by a CT scan before considering exploratory laparotomy. The CT scan provides accurate localization of the injury which allows more targeted, less invasive and more efficient interventions, potentially waiving the need for exploratory laparotomy.
Thoracic trauma
Most common cause of blunt thoracic trauma is motor vehicle accident (MVA) and fall accounting for up to 60% of the cases with a mortality rate of up to 10%, compared to overall trauma mortality of 4.3% (30). A portable chest radiograph is typically the first imaging study after blunt trauma with a primary goal to confirm supportive device placement and evaluate life threatening emergencies, such as a tension pneumothorax (31). Chest CT with IV contrast is the imaging study of choice, preferably MDCT (30). According to the most recent National Institute for Health and Care Excellence (NICE) guidelines, in adult patients (>16 years old) with chest injury and severe respiratory compromise, immediate chest X-ray is recommended. Extended focused assessment with sonography for trauma (eFAST) may be considered to augment the physical examination findings. eFAST may not be considered if a specialist team equipped with ultrasound is immediately not available or if it causes a delay in the transfer of the patient. In an adult patient with suspected chest trauma without severe respiratory compromise and hemodynamic stability, immediate chest CT is recommended. In children (<16 years old) chest X-ray and ultrasound should be used as first-line imaging (32).
In addition to the diagnostic information, ultrasound provides therapeutic opportunity in cases with traumatic pneumothorax and hemothorax. Traumatic pneumothorax results from penetrating/blunt trauma or iatrogenic secondary to thoracentesis, central venous line placement or lung biopsy. Ultrasound serves an effective guide in chest tube placement, resulting in lower incidence of complications, including iatrogenic pneumothorax and hemorrhage (33). The traditional anatomical teaching suggests that intercostal artery lies in the costal groove. However, recent studies show variation in the position and tortuosity of the vessel which only comes to lie in the subcostal groove towards the mid-axillary line (34).
Persistent intrathoracic hemorrhage and hemothorax is a frequent finding in patients with blunt or penetrating chest trauma. Exploratory laparotomy—the treatment of choice in hemodynamically unstable patients, while chest tube insertion with observation was the management of choice in hemodynamically stable patients.
Management of thoracic vascular injury such as traumatic aortic injury has evolved. Advances in imaging provide an accurate diagnosis in less time as well as endovascular management techniques. Development of stent graft technology provides definitive repair of the branch vessel and traumatic aortic injury (35).
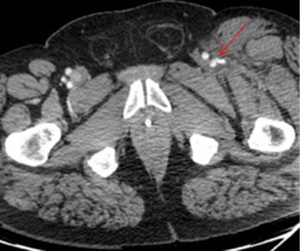
Intercostal artery injury, with or without rib fracture, is a frequent cause of intrathoracic hemorrhage in patients with blunt, penetrating or iatrogenic trauma. CT scan of the chest with IV contrast is the investigation of choice to identify the bleeding vessel. Selective and super-selective angiography is used for identification and embolization of intercostal arteries, which is performed safely with high success rates (Figures 3,4). The choice of the embolic agent is variable depending on the bleeding vessel caliber and familiarity of the operator. Mostly, a combination of coils or NBCA is used (36).
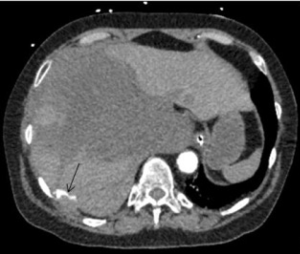
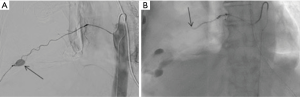
Microcatheter advancement and a selective angiogram are useful in identifying the anatomy and avoiding complications. Exact knowledge of anatomic variations in intercostal arteries is required to prevent complications. The most feared complication of embolization of intercostal arteries is spinal cord ischemia. The artery of Adamkiewicz supplying the anterior spinal artery may arise from the intercostal artery and if accidentally embolized, is known to cause motor paralysis from that level below (37).
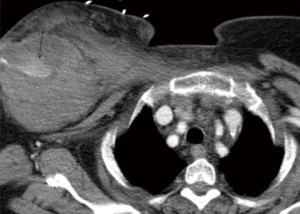
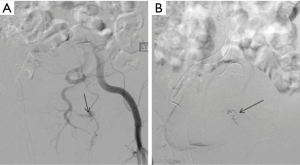
The subclavian and axillary arteries are prone to injury at the anatomic transition between the bony thorax and upper extremities. Endovascular therapy in management holds technical and physiological advantage compared to open surgical repair. Surgical approach requires deep and multiple incisions to access injured vessels. While, endovascular approach provides access to the injured vessel remotely from the same point of IV access crossing the zone of anatomic transition (Figures 5,6). This also avoids further exacerbation of injury by surgical dissection (38).
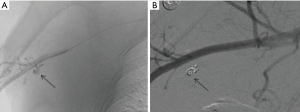
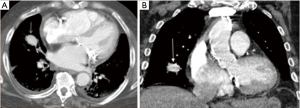
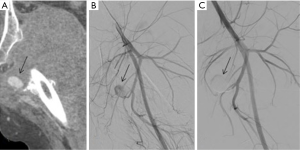
Pulmonary artery injury is the most feared iatrogenic traumatic complication caused by pulmonary artery catheter placement, often presenting with hemoptysis after the procedure. Endovascular coiling is the effective means of management (39) (Figures 7,8).

Abdominal trauma
The initial CT scan helps in grading the injury to abdominal organs according to American Association for the Surgery of Trauma (AAST) organ injury scale (40). The management is often dependent on the grade of trauma in addition to the hemodynamic status.
Splenic trauma
An unidentified splenic injury is the leading cause of preventable death in patients who’ve suffered a traumatic injury. The spleen is the most commonly injured organ in blunt abdominal trauma (41,42). Hemodynamically unstable patients or patients transiently responding to fluid resuscitation are treated with emergent laparotomy. Hemodynamically stable patients may be managed non-operatively with the use of IR (43). World Society of emergency surgeons recently published their workflow algorithm for patients with splenic trauma (44). However, practically the management varies depending on the facilities available, surgeon’s and interventional radiologist’s judgment, any additional trauma, initial clinical presentation, hemodynamic stability, response to initial fluid bolus, grade of splenic trauma and FAST findings. The decision to choose between IR and surgery is not solely dependent on hemodynamic stability and grade of splenic trauma. For example, at our institute, a grade 5 splenic trauma, even if hemodynamically stable, is taken for surgery, grade 1 splenic laceration and hemodynamically surgery, grade 3 liver laceration and hemodynamically stable should be treated with IR.
AAST validated the organ injury scoring for spleen trauma (Table 2) (40).

Full table
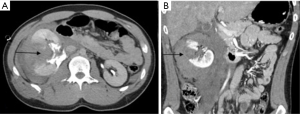
Contrast enhanced CT is the imaging modality of choice for characterization and grading of splenic injury according to the AAST. In addition to evaluation of the extent of hemoperitoneum, assessment of additional accompanying injuries can be made. Early CECT is imperative in triaging of the patient for operative vs. non-operative management. Intravenous contrast extravasation (ICE), arterial pseudoaneurysm (PSA) and AVF require urgent surgical or endovascular management (45). Although, the validity of these CECT findings and their correlation with angiography have been questionable in many studies (46,47).
The two most common techniques used in the management of splenic trauma are: proximal main splenic artery occlusion (SAO) and distal splenic branch embolization (SBE). SAO involves mechanical occlusion of the main splenic artery. While SBE includes super selective embolization of splenic artery branches distal to splenic hilum (2).
SAO is used in hemodynamically stable patients and is known to improve splenic salvage rates from 89% to 97% (48,49). It is indicated in patients with CECT showing either AAST grade 3 or higher splenic injury, moderate to large hemoperitoneum, or splenic ICE/PSA/AVF (Figure 9). It is also indicated in patients with known splenic trauma, with falling hematocrit requiring two or more units of transfusion in 24 hours or persistent tachycardia despite resuscitation. Mechanical occlusion of the main splenic artery lowers parenchymal blood pressure which hastens the healing process and prevents delayed splenic rupture. The blood flow to the spleen is maintained by collateral circulation including pancreatic, gastroepiploic, short gastric and splenic capsular vessels. This collateral perfusion prevents splenic infarction and preserves splenic function (2,45). SAO embolization is typically performed with metallic coils or vascular plugs 2 cm distal to the dorsal pancreatic artery. The post-procedural angiogram should show occlusion of the main splenic artery with delayed perfusion of splenic parenchyma via collaterals (2).
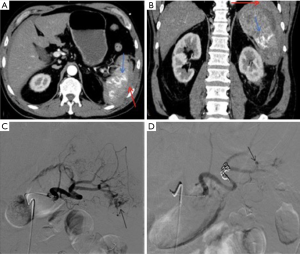
SBE is reserved for cases with a solitary focal splenic injury with positive angiographic findings (ICE, PSA or AVF). SBE requires the use of a micro-catheter for super selective catheterization. It is associated with higher procedure time, radiation exposure and contrast use. It is also associated with higher complication rates like splenic infarction, without any significant improvement in overall outcome (50). One advantage of SBE over SAO is it maintains the access for repeat embolization, if continued bleeding occurs via the collaterals.
SAO is more frequently done than SBE for control of splenic hemorrhage, with occasional use of both techniques in some patients with an overall success rate of 93.3% (28).
The failure rate of splenic embolization is higher in patients with high-grade trauma with moderate to large hemoperitoneum. The failure rate is approximately 10% requiring splenectomy (51,52). Overall complication rate with splenic embolization is 20%. Most complications are minor and include fever, left upper quadrant pain and left pleural effusion, managed supportively. Less commonly, patients can have continued bleeding, splenic artery dissection, splenic infarction, splenic abscess, contrast induced nephropathy, pancreatitis or coil migration into the non-target organ (53).
Hepatic trauma
Due to lack of randomized controlled trials for evaluation of effective arterial embolization in trauma, the Eastern Association for surgery of trauma guidelines offered level 2 recommendations for AE as a first line treatment of liver trauma in patients with transient response to resuscitation, in addition to potential operative intervention (54). AAST validated the organ injury scoring for hepatic trauma (Table 3).
Full table
Non-surgical management of blunt hepatic trauma is the standard of care and has a success rate of over 80% (55). Currently, hemodynamic instability is the only absolute contraindication to the non-surgical management. Earlier, numerous clinical factors were considered contraindicated for a trial of non-surgical management including: AAST grade 3 hepatic injury or greater, ICE on CT, moderate to large hemoperitoneum, age >55 years, impaired neurological status and requirement of multiple transfusions. In hemodynamically unstable patients, surgical interventions including hepatorrhaphy, non-anatomic resection, and peri-hepatic packing are often used (2).
Hepatic angiography and embolization (HAE) has high success rates in controlling arterial hemorrhage and is often used independently or to supplement surgical management.
CECT is recommended prior to HAE for localization and characterization of sites of injury in addition to delineate hepatic arterial anatomy. Hepatic artery anatomic variants are common with a reported incidence of 24% to 49%. The most common variants include: replaced/accessory right hepatic artery arising from the superior mesenteric artery and replaced/accessory left hepatic artery arising from the left gastric artery. These two variants make up to 90% of variant anatomy (56).
Hepatic arterial embolization is well tolerated in the presence of a patent portal vein due to its majority blood supply to the liver. Bile ducts are predominantly supplied by hepatic arteries; hence, embolization of central hepatic arteries is associated with subsequent risk of biliary necrosis (57). Endovascular management using covered stent placement is the treatment of choice in the management of hemorrhage from common or proper hepatic artery. The gastroduodenal artery may be presumptively embolized to prevent an endoleak before placing the stent. In cases requiring right hepatic artery embolization, it should be embolized distal to the origin of the cystic artery to avoid potential gallbladder ischemia/infarction or cholecystitis (58).
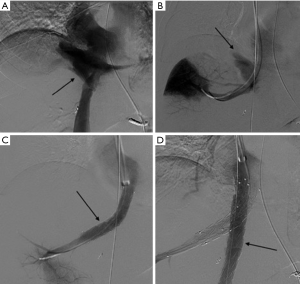
Hepatic arteries are not end-arteries and have good intra-hepatic collateralization, unlike spleen and kidney, the sandwich technique is frequently used (59). It involves the use of a co-axial micro-catheter system for embolization distal and proximal to the site of injury preventing distal reconstitution of an injured artery. If access distal to the point of injury is not feasible, selective distal embolization using gel-foam slurry or cyanoacrylate can be performed. Also, if the bleeding is multifocal in distribution a gel-foam slurry can be used for embolization. Coils and plugs are more useful for management of arterial fistulas or PSA (Figures 10-13).
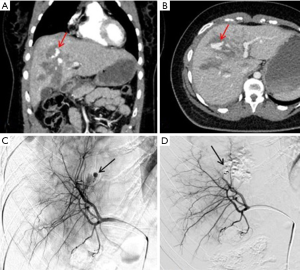


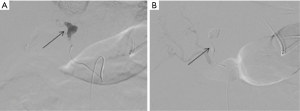
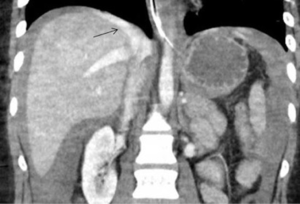
The incidence of hepatic venous injury is 13% in patients with liver trauma due to blunt abdominal injury. Hepatic venous injury is associated with almost double (61%) mortality compared to overall mortality with liver trauma (31%). The most common pattern of injury involves avulsion of the trunk of the right hepatic vein from inferior vena cava (IVC) or avulsion of the upper branch of the right hepatic vein (60). Management of hepatic venous trauma is usually surgical. Non-operative management options are insufficient, and no definitive endovascular treatment is described. At our center, we have placed an endovascular covered stent in the hepatic vein extending into the IVC, bridging the injury site. A second covered stent was placed in the IVC to assure cardio-pedal flow in the IVC across the hepatic vein-IVC stent site (Figures 14,15).
The incidence of post-HAE complication is increased with the AAST grade of initial hepatic injury. The overall reported complication rate is 61%. The most common complication is hepatic necrosis (42%) followed by hepatic abscess, gall bladder necrosis, and bile leak/biloma; in decreasing order of prevalence (57). Most of these complications (85%), can be managed non-operatively using conservative management, percutaneous drainage, ERCP or other interventional techniques (61).
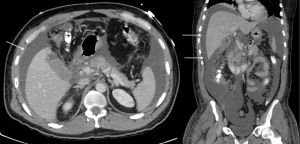
Traumatic bile duct injury is usually iatrogenic and is seen as a complication of hepatobiliary surgery (62). However, disruption of biliary tree secondary to blunt abdominal trauma is known to happen with a few reported cases in literature (63). These are often difficult to identify when associated with blunt abdominal trauma due to associated, immediate and more life-threatening injuries. Most cases of traumatic biliary injury are managed surgically. Recent advances in IR have made IR techniques an acceptable alternative in the management of biliary trauma. After gaining percutaneous access to the biliary tree, the guidewire is passed across the traumatic site, and a covered stent is subsequently advanced over the guidewire and is then secured in position (Figures 16-20).
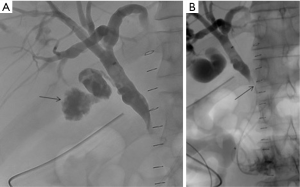
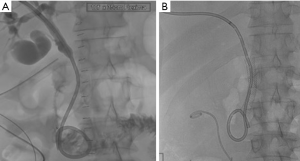
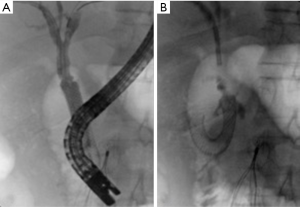
Renal trauma
The incidence of renal injury in blunt abdominal trauma is 10%, making kidney the 3rd most commonly injured organ (2). Nonsurgical management is the treatment of choice in hemodynamically stable patients since, in addition to avoiding surgical morbidity, it preserves renal function. Most commonly, traumatic renal injury is low grade (AAST grade 1 and grade 2) (Table 4) and management is conservative with observation (64). High-grade renal trauma (AAST grade 3, 4 and 5) are managed with intervention or surgery. However, if hemodynamically stable, a trial of non-operative management may be considered. Studies have shown the safety and efficacy of observation and adjunctive AE for high-grade renal trauma with intermediate-term follow-up results (2). AAST organ injury scales for Kidney trauma (Table 4) (40).
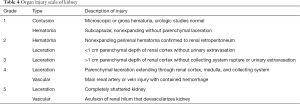
Full table
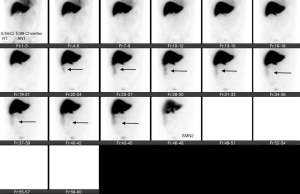
The goal of AE in high-grade renal trauma is arresting hemorrhage with preservation of renal function. Hence, embolization needs to be selective. Most studies have demonstrated technical success rate of endovascular management of renal trauma at 96.2% and clinical success rate of 90.92% with a high re-bleeding rate of 25.6% (28). Endovascular management of grade 5 renal trauma is controversial with success rate varying from 0% to 100% (65,66).
CECT with nephrographic and excretory phase is the gold standard for assessment of renal and ureteral trauma. CECT demonstrates ICE, AFV, and PSA. In addition to the AAST grading, CECT also shows the extent and location of de-vascularized renal parenchyma. The indications for AE in renal trauma include ICE, AFV, PSA, persistent gross hematuria, hemodynamic instability or falling hematocrit (65).
A flush aortogram before renal artery catheterization is helpful in delineating the arterial anatomy and identifying any accessory renal arteries that might be the source of bleeding. Positive angiographic findings include ICE, PSA, and AVF, occlusion, dissection and arteriocalyceal fistula. Sub-selective embolization using micro-catheter is typically performed using coils and/or gel-foam. A less selective catheterization can be conducted using coils and gel-foam slurry if the injured vessel cannot be embolized (2).
Main renal artery trauma is rarely secondary to blunt abdominal trauma, with an incidence of 0.05% (67). Treatment is controversial and could involve nephrectomy or vascular repair (68). However, endovascular management using bare metal or stent graft placement may be considered in individual appropriate cases. In patients with successful recanalization of occluded and dissected main renal artery, long term salvage is not always achieved. Some patients developed reno-vascular hypertension requiring nephrectomy (69).
Endovascular management of large vessel injury requires the use of stent/graft placement to cover the site of bleeding (2) (Figure 21). Small hemorrhages from distal branches of the hepatic artery are managed by coiling (Figures 22,23).

Pelvic injury
The pelvic fractures are seen in approximately 4–9% of patients with blunt trauma (70). Lateral compression fracture is the most common type of injury in the pelvis and constitutes nearly 65% of pelvic fractures. Lateral compression fractures are usually stable without any ligamentous injury and require angiography in only 1% of cases. Anteroposterior, vertical shear and combined types of pelvic fractures are often associated with ligamentous injuries and an increase in pelvic volume. The need for angiography, in this case, is around 20% (71). Emergent pelvic AE is suggested in hemodynamically unstable patients with pelvic fractures.
Increase in the pelvic volume removes the tamponading effect of the intact pelvis and leads to threatening hemorrhage. Diastasis of pubic symphysis by 3 cm can double the pelvic volume (72). Hemodynamic stability plays a significant role in overall mortality in pelvic trauma patients. Hemodynamically stable patients have less than 5% mortality while hemodynamically unstable patients have mortality rate up to 40% (73). In many cases, bleeding is venous in origin and is caused by bone fracture edges, soft tissue, and veins. Venous bleeding can often be controlled by external stabilization of the pelvis. AE is used exclusively for controlling pelvic arterial hemorrhage with a success rate of 95% and a low complication rate (74).
Since surgical options are limited in pelvic hemorrhage, AE is recommended. IR techniques are used in patients with active arterial pelvic hemorrhage unless immediate open surgery is not required to control bleeding from other injuries (32).
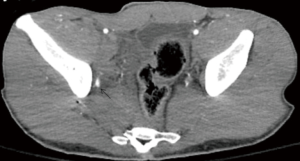
A significant survival benefit is seen if embolization is performed within 3 hours in patients with pelvic fracture and arterial bleeding (75). Emergent pelvic AE is recommended if the patient is hemodynamically unstable with a pelvic fracture(s) and no other sources of bleeding or if the patient has evidence of active extravasation on contrast enhanced CT regardless of hemodynamic stability (76,77). Pelvic CTA is useful in localization and characterization of pelvic hemorrhage in addition to identifying concomitant injuries in up to 50% of patients (78). ICE on CTA when compared to angiography as a gold standard, has a 90.5% sensitivity and 96.1% specificity for arterial bleeding (79). CTA of the pelvis can be easily integrated into the trauma CT scans of chest and abdomen (80).
Trauma patients with pelvic fracture usually arrive at the angiography suite with a pelvic binder in place. A notch must be cut in the binder to allow access to the femoral artery without compromising the binders’ compressive effect. Alternate access via brachial or radial artery may be obtained if femoral artery access is difficult. Femoral access contralateral to the side of injury is preferred due to favorable catheterization angles and reduced catheter overlap that might obscure area of interest (2).
Pelvic angiogram for trauma should include an aortogram of infra-renal aorta followed by selective angiogram of internal iliac arteries. Pelvic arterial injury most commonly involves internal iliac branches. Although, selective external iliac artery angiogram is recommended when internal iliac artery angiogram is negative, and there is a high suspicion of continuing pelvic arterial hemorrhage. Selective external iliac artery angiography is recommended when ICE is present on a CECT (81).
In hemodynamically unstable patients with an internal iliac artery source, embolization of bilateral internal iliac arteries with gel-foam pledgets or slurry is recommended and is known to be very effective in achieving hemostasis (46).
In hemodynamically stable patients with a limited number of bleeding foci, selective embolization using coaxial micro-catheters, coils and/or gel-foam is indicated. Post procedure angiogram of the contralateral internal iliac arteries is recommended to exclude any possible bleeding. If present, the bleeding vessels are also embolized. In patients with positive CECT and without any evidence of angiographic correlate, a non-selective embolization of the entire anterior or posterior division of the internal iliac artery is performed as identified on the CECT.
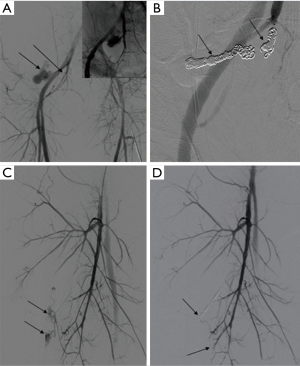
The most commonly injured pelvic arteries in decreasing order of frequency are: superior gluteal, internal pudendal, obturator, inferior gluteal, lateral sacral, iliolumbar, external iliac, deep circumflex iliac, and inferior epigastric arteries (71). Pelvic arterial trauma includes ICE, PSA, AVF, dissection, transection, and occlusion. The goal of AE is occlusion of the injured vessel and in most cases, gel-foam is used. However, in PSA and AVF management coils and plugs are preferred. The operator needs to be cautious in the management of AVF since there is a potential for coil migration through the fistula into the pulmonary circulation.
The overall clinical success rate of 91.75% is reported in the literature (28) for pelvic arterial embolization. Persistent or delayed hemorrhage after the AE is the most worrisome complication with an incidence of 15–20% and is associated with increased mortality (82) (Figures 24-27).
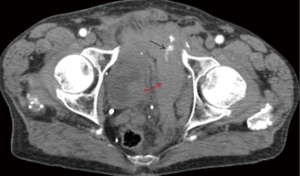
There no evidence of increased incidence of male impotence or gluteal necrosis due to pelvic embolization, contrary to early reports (83,84).
There are various limitations associated with AE. An occluded arterial branch may reflect vasospasm secondary to distal vessel injury. Arterial spasm may resolve with time and resuscitation and eventually cause delayed hemorrhage, if not treated. Also, angiographic false positives include: bowel and ureteral peristalsis and uterine and cavernosal blush (2).
Major vascular injury and extremity trauma
NICE’s recent recommendations on the initial assessment and management of major trauma are as follows. Immediate CT must be considered in patients with suspected hemorrhage if they are adequately responding to resuscitation and if their hemodynamic status is normal. In patients who are not respond to volume resuscitation, diagnostic imaging such as chest and pelvic X-rays or FAST should be limited. Since a negative FAST does not exclude intra-abdominal, intraperitoneal or retroperitoneal hemorrhage. An immediate CT is recommended instead of FAST (32).
In patients with blunt trauma and multiple suspected injuries, a whole body “scanogram”, which is routinely obtained before a CT scan, can be extended to cover the body from vertex to mid thighs to evaluate for any limb fracture. The “scanogram” should be used to direct additional CT scanning of the limbs if required (32).
Penetrating visceral and extremity trauma is associated with higher incidence of vascular injury. In penetrating extremity trauma, the most commonly injured vessels are muscular branches of the deep femoral artery (85). Penetrating injury to the profunda femoris artery is known to cause a PSA with or without active extravasation (86). The traditional management is a surgical repair. But, it is often made difficult by the associated hematoma and active bleeding limiting access into the deep compartment of the thigh. Endovascular treatment options vary from the use the use of onyx to pledgets and coils. Selective catheterization of the PFA allows embolization of the artery immediately distal and then proximal to the neck of an aneurysm (Figure 28). In some cases, a covered stent can also be used to jail-off the aneurysm while preserving blood supply to the distal lower extremity (Figures 28-30).
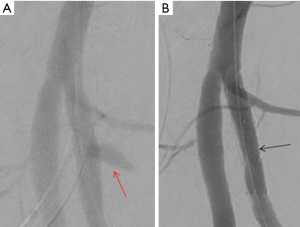
Conclusions
Traumatic injuries, being a common cause of significant mortality and morbidity, can be successfully managed non-operatively with adjunctive IR techniques, which are the effective first line therapy in hemodynamically stable patients. AE and other IR techniques are used to improve the overall clinical outcomes with low procedural and post-procedural complication rates.
Effective integration of IR with surgery, interventional neuroradiology and emergency medicine requires adequate staffing, organized multidisciplinary evaluation and direct communication with quick response times.
Customized hybrid operating rooms equipped for resuscitation, angiographic intervention, imaging capability, and surgical management of trauma patients are becoming the wave of the future for delivering expedited multi-disciplinary care.
Acknowledgments
We wish to acknowledge the effort and contributions of Kari D. Hopfer, DO; Hammed Ninawolo, MD.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Papakostidis C, Kanakaris N, Dimitriou R, et al. The role of arterial embolization in controlling pelvic fracture haemorrhage: a systematic review of the literature. Eur J Radiol 2012;81:897-904. [Crossref] [PubMed]
- McCabe S, Maddineni S, Marini C, et al. Vascular and Interventional Radiology in Blunt Abdominopelvic Trauma-Institutional Practice and Review of the Literature. J Trauma Treat 2016;5:324. [Crossref]
- Evans JA, van Wessem KJ, McDougall D, et al. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg 2010;34:158-63. [Crossref] [PubMed]
- Hudorović N, Rogan SA, Lovricević I, et al. The vascular hybrid room--operating room of the future. Acta Clin Croat 2010;49:289-98. [PubMed]
- Ott S, Struffert T, Hertel V, et al. Visualization and monitoring of acute epistaxis during endovascular treatment using a flat detector CT. Minim Invasive Neurosurg 2011;54:187-90. [Crossref] [PubMed]
- Hausegger KA, Fürstner M, Hauser M, et al. Clinical application of flat-panel CT in the angio suite. Rofo 2011;183:1116-22. [Crossref] [PubMed]
- Tonkopi E, Al-Habsi AH, Shankar JJ. Radiation Dose from 3D Rotational vs. Conventional 2D Digital Subtraction Angiography in Intracranial Aneurysm Coiling. Can J Neurol Sci 2015;42:176-80. [Crossref] [PubMed]
- Gupta R, Cheung AC, Bartling SH, et al. Flat-Panel Volume CT: Fundamental Principles, Technology, and Applications. Radiographics 2008;28:2009-22. [Crossref] [PubMed]
- Ringelstein A, Mueller O, Mönninghoff C, et al. 3D Rotational Angiography After Non-Traumatic SAH. Rofo 2014;186:675-9. [Crossref] [PubMed]
- Banerjee A, Zosa BM, Allen D, et al. Implementation of an image sharing system significantly reduced repeat computed tomographic imaging in a regional trauma system. J Trauma Acute Care Surg 2016;80:51-4; discussion 54-6. [Crossref] [PubMed]
- Joslin JD, Goldberger D, Johnson L, et al. Use of the Vocera Communications Badge Improves Public Safety Response Times. Emerg Med Int 2016;2016:7158268.
- McKnight R, Franko O. HIPAA Compliance with Mobile Devices Among ACGME Programs. J Med Syst 2016;40:129. [Crossref] [PubMed]
- Frevert S, Dahl B, Lönn L. Update on the roles of angiography and embolisation in pelvic fracture. Injury 2008;39:1290-4. [Crossref] [PubMed]
- Idle MR, Monaghan AM, Lamin SM, et al. N-butyl-2-cyanoacrylate (NBCA) tissue adhesive as a haemostatic agent in a venous malformation of the mandible. Br J Oral Maxillofac Surg 2013;51:565-7. [Crossref] [PubMed]
- Van Ha TG. Use of the Interlock Fibered IDC Occlusion System in Clinical Practice. Semin Intervent Radiol 2008;25:3-10. [Crossref] [PubMed]
- Rossi G, Mavrogenis AF, Rimondi E, et al. Selective embolization with N-butyl cyanoacrylate for metastatic bone disease. J Vasc Interv Radiol 2011;22:462-70. [Crossref] [PubMed]
- Kominami S, Watanabe A, Suzuki M, et al. Preoperative embolization of meningiomas with N-butyl cyanoacrylate. Interv Neuroradiol 2012;18:133-9. [Crossref] [PubMed]
- Igarashi S, Izuchi S, Ogawa Y, et al. N-Butyl Cyanoacrylate Is Very Effective for Massive Haemorrhage during the Perinatal Period. PLoS One 2013;8:e77494. [Crossref] [PubMed]
- Choi JW, Kim HC, Jae HJ, et al. Transcatheter Embolotherapy with N-Butyl Cyanoacrylate for Ectopic Varices. Cardiovasc Intervent Radiol 2015;38:344-51. [Crossref] [PubMed]
- Pietura R, Toborek M, Dudek A, et al. Endovascular embolization of varicoceles using n-butyl cyanoacrylate (NBCA) glue. Pol J Radiol 2013;78:26-30. [Crossref] [PubMed]
- Kolber MK, Shukla PA, Kumar A, et al. Ethylene Vinyl Alcohol Copolymer (Onyx) Embolization for Acute Hemorrhage: A Systematic Review of Peripheral Applications. J Vasc Interv Radiol 2015;26:809-15. [Crossref] [PubMed]
- Andaluz N, Zuccarello M. Recent trends in the treatment of cerebral aneurysms: analysis of a nationwide inpatient database. J Neurosurg 2008;108:1163-9. [Crossref] [PubMed]
- Guglielmi G, Viñuela F, Sepetka I, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: Electrochemical basis, technique, and experimental results. J Neurosurg 1991;75:1-7. [Crossref] [PubMed]
- Hui FK, Fiorella D, Masaryk TJ, et al. A history of detachable coils: 1987-2012. J Neurointerv Surg 2014;6:134-8. [Crossref] [PubMed]
- Malisch TW, Guglielmi G, Viñuela F, et al. Intracranial aneurysms treated with the Guglielmi detachable coil: midterm clinical results in a consecutive series of 100 patients. J Neurosurg 1997;87:176-83. [Crossref] [PubMed]
- Kleine JF, Prothmann S, Boeckh-Behrens T. Occlusion of Small Arteries in the Neuroendovascular and Head and Neck Territory—Initial Experiences with a Microvascular Plug. J Vasc Interv Radiol 2015;26:426-31. [Crossref] [PubMed]
- Roy-Choudhury SH, Gallacher DJ, Pilmer J, et al. Relative Threshold of Detection of Active Arterial Bleeding: In Vitro Comparison of MDCT and Digital Subtraction Angiography. AJR Am J Roentgenol 2007;189:W238-46. [Crossref] [PubMed]
- Ierardi AM, Duka E, Lucchina N, et al. The role of interventional radiology in abdominopelvic trauma. Br J Radiol 2016;89:20150866. [Crossref] [PubMed]
- Zhang Z, Hong Y, Liu N, et al. Diagnostic accuracy of contrast enhanced ultrasound in patients with blunt abdominal trauma presenting to the emergency department: a systematic review and meta-analysis. Sci Rep 2017;7:4446. [Crossref] [PubMed]
- Sridhar S, Raptis C, Bhalla S. Imaging of Blunt Thoracic Trauma. Semin Roentgenol 2016;51:203-14. [Crossref] [PubMed]
- Ho ML, Gutierrez FR. Chest Radiography in Thoracic Polytrauma. AJR Am J Roentgenol 2009;192:599-612. [Crossref] [PubMed]
- Glen J, Constanti M, Brohi K. Assessment and initial management of major trauma: summary of NICE guidance. BMJ 2016;353:i3051. [Crossref] [PubMed]
- McDermott S, Levis DA, Arellano RS. Chest drainage. Semin Intervent Radiol 2012;29:247-55. [Crossref] [PubMed]
- Dewhurst C, O’Neill S, O’Regan K, et al. Demonstration of the course of the posterior intercostal artery on CT angiography: relevance to interventional radiology procedures in the chest. Diagn Interv Radiol 2012;18:221-4. [PubMed]
- Malloy PC, Richard HM 3rd. Thoracic angiography and intervention in trauma. Radiol Clin North Am 2006;44:239-49. [Crossref] [PubMed]
- Chemelli AP, Thauerer M, Wiedermann F, et al. Transcatheter arterial embolization for the management of iatrogenic and blunt traumatic intercostal artery injuries. J Vasc Surg 2009;49:1505-13. [Crossref] [PubMed]
- Finstein JL, Chin KR, Alvandi F, et al. Postembolization paralysis in a man with a thoracolumbar giant cell tumor. Clin Orthop Relat Res 2006.335-40. [Crossref] [PubMed]
- Gilani R, Tsai PI, Wall MJ Jr, et al. Overcoming challenges of endovascular treatment of complex subclavian and axillary artery injuries in hypotensive patients. J Trauma Acute Care Surg 2012;73:771-3. [Crossref] [PubMed]
- Nellaiyappan M, Omar HR, Justiz R, et al. Pulmonary artery pseudoaneurysm after Swan-Ganz catheterization: a case presentation and review of literature. Eur Heart J Acute Cardiovasc Care 2014;3:281-8. [Crossref] [PubMed]
- Tinkoff G, Esposito TJ, Reed J, et al. American Association for the Surgery of Trauma Organ Injury Scale I: Spleen, Liver, and Kidney, Validation Based on the National Trauma Data Bank. J Am Coll Surg 2008;207:646-55. [Crossref] [PubMed]
- Schroeppel TJ, Croce MA. Diagnosis and management of blunt abdominal solid organ injury. Curr Opin Crit Care 2007;13:399-404. [Crossref] [PubMed]
- Cales RH, Trunkey DD. Preventable trauma deaths. A review of trauma care systems development. JAMA 1985;254:1059-63. [Crossref] [PubMed]
- Sartorelli KH, Frumiento C, Rogers FB, et al. Nonoperative management of hepatic, splenic, and renal injuries in adults with multiple injuries. J Trauma 2000;49:56-61; discussion 61-2. [Crossref] [PubMed]
- Coccolini F, Montori G, Catena F, et al. Splenic trauma: WSES classification and guidelines for adult and pediatric patients. World J Emerg Surg 2017;12:40. [Crossref] [PubMed]
- Dent D, Alsabrook G, Erickson BA, et al. Blunt splenic injuries: high nonoperative management rate can be achieved with selective embolization. J Trauma 2004;56:1063-7. [Crossref] [PubMed]
- Raikhlin A, Baerlocher MO, Asch MR, et al. Imaging and transcatheter arterial embolization for traumatic splenic injuries: review of the literature. Can J Surg 2008;51:464-72. [PubMed]
- Haan JM, Biffl W, Knudson MM, et al. Splenic embolization revisited: a multicenter review. J Trauma 2004;56:542-7. [Crossref] [PubMed]
- Willmann JK, Roos JE, Platz A, et al. Multidetector CT: Detection of Active Hemorrhage in Patients with Blunt Abdominal Trauma. AJR Am J Roentgenol 2002;179:437-44. [Crossref] [PubMed]
- Marmery H, Shanmuganathan K, Mirvis SE, et al. Correlation of Multidetector CT Findings with Splenic Arteriography and Surgery: Prospective Study in 392 Patients. J Am Coll Surg 2008;206:685-93. [Crossref] [PubMed]
- Imbrogno BF, Ray CE. Splenic artery embolization in blunt trauma. Semin Intervent Radiol 2012;29:147-9. [Crossref] [PubMed]
- Smith HE, Biffl WL, Majercik SD, et al. Splenic artery embolization: Have we gone too far? J Trauma 2006;61:541-4. [Crossref] [PubMed]
- Schnüriger B, Inaba K, Konstantinidis A, et al. Outcomes of Proximal Versus Distal Splenic Artery Embolization After Trauma: A Systematic Review and Meta-Analysis. J Trauma 2011;70:252-60. [Crossref] [PubMed]
- Ekeh AP, McCarthy MC, Woods RJ, et al. Complications arising from splenic embolization after blunt splenic trauma. Am J Surg 2005;189:335-9. [Crossref] [PubMed]
- Stassen NA, Bhullar I, Cheng JD, et al. Nonoperative management of blunt hepatic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg 2012;73:S288-93. [Crossref] [PubMed]
- Velmahos GC, Toutouzas KG, Radin R, et al. Nonoperative treatment of blunt injury to solid abdominal organs: a prospective study. Arch Surg 2003;138:844-51. [Crossref] [PubMed]
- Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 1994;220:50-2. [Crossref] [PubMed]
- Dabbs DN, Stein DM, Scalea TM. Major Hepatic Necrosis: A Common Complication After Angioembolization for Treatment of High-Grade Liver Injuries. J Trauma 2009;66:621-7; discussion 627-9. [Crossref] [PubMed]
- Mohr AM, Lavery RF, Barone A, et al. Angiographic Embolization for Liver Injuries: Low Mortality, High Morbidity. J Trauma 2003;55:1077-81; discussion 1081-2. [Crossref] [PubMed]
- Mays ET 2nd, Mays ET. Are hepatic arteries end-arteries? J Anat 1983;137:637-44. [PubMed]
- Hollands MJ, Little JM. Hepatic venous injury after blunt abdominal trauma. Surgery 1990;107:149-52. [PubMed]
- Carrillo EH, Spain DA, Wohltmann CD, et al. Interventional techniques are useful adjuncts in nonoperative management of hepatic injuries. J Trauma 1999;46:619-22; discussion 622-4. [Crossref] [PubMed]
- Huang ZQ, Huang XQ. Changing patterns of traumatic bile duct injuries: a review of forty years experience. World J Gastroenterol 2002;8:5-12. [Crossref] [PubMed]
- Sanford Z, Abdolmaali K, Robinson D, et al. Blunt trauma: An uncommon cause of common bile duct injury. Trauma Case Reports 2015;1:44-8. [Crossref]
- Santucci RA, Wessells H, Bartsch G, et al. Evaluation and management of renal injuries: consensus statement of the renal trauma subcommittee. BJU Int 2004;93:937-54. [Crossref] [PubMed]
- Breyer BN, McAninch JW, Elliott SP, et al. Minimally invasive endovascular techniques to treat acute renal hemorrhage. J Urol 2008;179:2248-52; discussion 2253. [Crossref] [PubMed]
- Brewer ME Jr, Strnad BT, Daley BJ, et al. Percutaneous embolization for the management of grade 5 renal trauma in hemodynamically unstable patients: initial experience. J Urol 2009;181:1737-41. [Crossref] [PubMed]
- Sangthong B, Demetriades D, Martin M, et al. Management and Hospital Outcomes of Blunt Renal Artery Injuries: Analysis of 517 Patients from the National Trauma Data Bank. J Am Coll Surg 2006;203:612-7. [Crossref] [PubMed]
- Elliott SP, Olweny EO, McAninch JW, et al. Renal arterial injuries: a single center analysis of management strategies and outcomes. J Urol 2007;178:2451-5. [Crossref] [PubMed]
- Lopera JE, Suri R, Kroma G, et al. Traumatic occlusion and dissection of the main renal artery: endovascular treatment. J Vasc Interv Radiol 2011;22:1570-4. [Crossref] [PubMed]
- Demetriades D, Karaiskakis M, Toutouzas K, et al. Pelvic fractures: epidemiology and predictors of associated abdominal injuries and outcomes. J Am Coll Surg 2002;195:1-10. [Crossref] [PubMed]
- Kaufman JA, Lee MJ. Vascular and interventional radiology. Saunders, 2013:597.
- Agnew SG. Hemodynamically unstable pelvic fractures. Orthop Clin North Am 1994;25:715-21. [PubMed]
- Naam NH, Brown WH, Hurd R, et al. Major pelvic fractures. Arch Surg 1983;118:610-6. [Crossref] [PubMed]
- Velmahos GC, Toutouzas KG, Vassiliu P, et al. A prospective study on the safety and efficacy of angiographic embolization for pelvic and visceral injuries. J Trauma 2002;53:303-8; discussion 308. [Crossref] [PubMed]
- Agolini SF, Shah K, Jaffe J, et al. Arterial embolization is a rapid and effective technique for controlling pelvic fracture hemorrhage. J Trauma 1997;43:395-9. [Crossref] [PubMed]
- Cullinane DC, Schiller HJ, Zielinski MD, et al. Eastern Association for the Surgery of Trauma practice management guidelines for hemorrhage in pelvic fracture--update and systematic review. J Trauma 2011;71:1850-68. [Crossref] [PubMed]
- Yoon W, Kim JK, Jeong YY, et al. Pelvic arterial hemorrhage in patients with pelvic fractures: detection with contrast-enhanced CT. Radiographics 2004;24:1591-605; discussion 1605-6. [Crossref] [PubMed]
- Suzuki T, Smith WR, Moore EE, et al. Pelvic packing or angiography: competitive or complementary? Injury 2009;40:343-53. [Crossref] [PubMed]
- Brasel KJ, Pham K, Yang H, et al. Significance of contrast extravasation in patients with pelvic fracture. J Trauma 2007;62:1149-52. [Crossref] [PubMed]
- Anderson SW, Soto JA, Lucey BC, et al. Blunt trauma: feasibility and clinical utility of pelvic CT angiography performed with 64-detector row CT. Radiology 2008;246:410-9. [Crossref] [PubMed]
- Johnson GE, Sandstrom CK, Kogut MJ, et al. Frequency of external iliac artery branch injury in blunt trauma: improved detection with selective external iliac angiography. J Vasc Interv Radiol 2013;24:363-9. [Crossref] [PubMed]
- Fang JF, Shih LY, Wong YC, et al. Repeat transcatheter arterial embolization for the management of pelvic arterial hemorrhage. J Trauma 2009;66:429-35. [Crossref] [PubMed]
- Takahira N, Shindo M, Tanaka K, et al. Gluteal muscle necrosis following transcatheter angiographic embolisation for retroperitoneal haemorrhage associated with pelvic fracture. Injury 2001;32:27-32. [Crossref] [PubMed]
- Ramirez JI, Velmahos GC, Best CR, et al. Male sexual function after bilateral internal iliac artery embolization for pelvic fracture. J Trauma 2004;56:734-9; discussion 739-41. [Crossref] [PubMed]
- Kaufman JA, Parker JE, Gillespie DL, et al. Arteriography for proximity of injury in penetrating extremity trauma. J Vasc Interv Radiol 1992;3:719-23. [Crossref] [PubMed]
- Karkos CD, Karamanos DG, Papazoglou KO, et al. Ruptured pseudoaneurysm of the profunda femoris artery due to pellet injury: endovascular treatment by coil embolization. Cardiovasc Intervent Radiol 2009;32:837-9. [Crossref] [PubMed]
Cite this article as: Jeph S, Ahmed S, Bhatt RD, Nadal LL, Bhanushali A. Novel use of interventional radiology in trauma. J Emerg Crit Care Med 2017;1:40.


