
Interventional and operative procedures in complex and poly-trauma
Introduction
The initial evaluation for each trauma patient is dictated by Advanced Trauma Life Support (ATLS®) (1), but as the complexity of the patient’s injuries compound, the management becomes more complicated. Historically the approaches were invasive but straightforward—open the appropriate body cavity, identify the bleeding organ, and repair the damage. The high morbidity and mortality garnered by this approach has created the evolution of new techniques and ideas in trauma. From the resuscitative thoracotomy to the resuscitative endovascular balloon occlusion of the aorta (REBOA); additions such as preperitoneal pelvic packing to the algorithm of management of pelvic fractures; and the refinement of damage control laparotomy; trauma surgery has continued to expand beyond the standard scope of traditional general surgery to become a multi-disciplinary specialty with its own lexicon of interventional and operative procedures.
Ultrasound
Initial evaluation of a trauma patient follows the ATLS protocol: ABCDE (airway, breathing, circulation, disability, and exposure). The secondary survey looks for injuries and localizes pain, and imaging is taken into account. Point-of-care ultrasound has become a standard component of the secondary survey since it is noninvasive, sensitive in expert hands, and rapid to perform. Extended focused assessment with sonography for trauma (eFAST) focuses on identifying life-threatening torso hemorrhage (2,3) or pneumothorax by scanning the pericardial sac, right upper quadrant, left upper quadrant, pelvis, and lungs (4). It carries an acceptable sensitivity and specificity (92–97% and 93–99% respectively) (5-7). All areas of interest should be scanned completely using a sweeping motion in multiple planes. In multiple studies, including randomized trials, FAST decreased the time to OR (2,8) allowing more patients to be treated in the “golden hour” (9). Further evaluation of trauma patients using contrast enhanced ultrasound (CEUS) has shown promise in diagnosing solid organ injury (10), but currently CT remains the gold standard for evaluation and grading of these injuries (11). Additionally, it should be noted that retroperitoneal injuries are not well visualized on ultrasound (12), and thus CT is still typically performed. Procedures performed in the trauma bay, such as femoral central venous or arterial line placement are facilitated by ultrasound, much as US guided line placement is becoming standard in the ICU setting (13).
Resuscitative endovascular balloon occlusion of the aorta
Emergency Department Thoracotomy (EDT) is used to rescue the trauma patient who arrives in cardiac arrest or arrests shortly after arrival to the emergency department. A left anterolateral thoracotomy incision is made, the pericardial sac is opened, open cardiac massage is performed and the thoracic aorta is cross clamped to maximize perfusion in the coronary and cerebral vascular systems and stop the outflow to potential bleeding in the abdomen or pelvis (14). EDT is a maximally invasive procedure with high mortality between 70% and 99% (15). This has shown the most benefit for patients with penetrating cardiac injuries, but may still be indicated for patients suffering blunt trauma who progress to cardiac arrest with a controllable sub-diaphragmatic process (16). Some debate the utility of such a procedure as it seems to carry a poor survival and puts providers at risk for injury. Balloon occlusion of the aorta has recently been revisited as a less invasive intervention to replace resuscitative thoracotomy (17,18). This technique was initially pioneered for use in ruptured abdominal aortic aneurysm (19). Over the last several years, REBOA kits have become increasingly available. Reports show it may provide similar physiologic improvement to aortic cross clamping (20). In addition, REBOA can be used earlier than EDT (21). While thoracotomy is often employed only once a patient has lost vital signs, because of its less invasive nature REBOA can be used pre-arrest in the hypotensive patient. Earlier use of this occlusive balloon “cross clamp” may prevent physiologic derangement and improve mortality.
While there is no standardized training for REBOA placement, several commercial courses are available including the Endovascular Skills for Trauma and Resuscitative Surgery (ESTARS) Course (22), and the BEST (Basic endovascular Skills for Trauma) course (23). Placing the REBOA catheter involves: (I) obtaining femoral arterial access via a cut-down or percutaneous method; (II) inserting the catheter to the appropriate location; and (III) inflating the balloon until the appropriate resistance achieved (21,24). Chest or abdominal radiographs confirm correct position. There is a now commercially available ER-REBOA device (Prytime Medical, Boerne, TX) that does not require a guidewire and has a 7F sheath as opposed to the 12F sheath previously required (25). The smaller sheath allows for removal with a closure device or simple pressure where previously an arterial repair was necessary (26).
Patient selection for REBOA has typically been reserved for patients who present with a SBP <90 mmHg despite adequate resuscitation efforts, and a positive FAST exam or pelvic fracture. REBOA is contraindicated in patients who have suspected or confirmed intrathoracic injury, as thoracotomy would provide direct hemorrhage control. The choice for position of the balloon depends on the site of exsanguination. Zone 1 is between the takeoff of the left subclavian artery and the celiac artery. Indications for placement in this zone include intraabdominal hemorrhage indicated by a positive FAST exam. Zone 2 begins at the celiac artery and ends at the lower renal artery. There is no indication for REBOA in this area. Zone 3 encompasses the lower renal artery to the aortic bifurcation, and is utilized in the control of pelvic hemorrhage (17). Correct placement has been seen to raise systolic blood pressure by 40–70 mmHg (23,27) allowing time to obtain definitive hemorrhage control (18,23,27).
Duration of safe aortic occlusion is estimated to be 40 minutes (28). Newer methods of overcoming this time frame are being explored including intermittent balloon deflation allowing for temporary blood flow every 20 minutes (29) and partial REBOA (P-REBOA) where the balloon is deflated just enough to allow a 10 mm Hg rise in the MAP distal to the balloon (30). Both these options offload supraphysiologic proximal pressure and reduce the stress on the heart due to increased afterload, and decrease distal ischemia.
Once the REBOA catheter is in place and the patient has stabilized, choice of definitive hemorrhage control depends on the suspected or known injury, resources of the facility, and clinical decision making by surgeon. The patient should not leave the operating room until definitive surgical hemostasis has been obtained and the balloon has been removed. Balloon deflation should be done slowly over the course of several minutes, with communication to the resuscitative team to prepare for hypotension and acidosis from reperfusion (31).
Certain studies have attempted to compare REBOA to EDT (31,32). Moore et al. in 2015 looked at 24 REBOA and 74 EDT patients and noted an overall survival rate of 37.5% vs. 9.7% (32). However, 71% of the REBOA patients had vital signs present at ED admission as compared to 38% in the EDT group. This suggests that REBOA was utilized earlier in those less physiologically deranged. While earlier use is one of the benefits of REBOA, it makes comparison to EDT difficult. The same group subsequently looked at retrospective data and found that though patients treated with REBOA had an overall survival of 32%, those who had CPR in progress at the time of REBOA placement only survived to discharge 10% of the time (21). While there does appear to be some efficacy of REBOA, there has yet to be high quality data comparing the two. In addition, these studies were conducted at high volume level 1 trauma centers, and it remains to be seen if these experiences are generalizable to other institutions with fewer immediately available resources.
Several large series have published complications associated with the REBOA technique, most of which have to do with the femoral access site (33,34). These include AV fistulas, pseudoaneurysms, and arterial rupture (35). In one series 3 patients of 24 developed lower extremity ischemia requiring amputation (36). Non-access site complications include abdominal organ ischemia, aortic injuries and misplaced balloons. When REBOA is used as a lifesaving measure, rare complications may be acceptable but as this technique is used in the less moribund patient its benefit must clearly outweigh the risk.
Complex pelvic fractures
Severe pelvic fractures with hemorrhage represent a unique multispecialty problem involving trauma surgery, interventional radiology and orthopedics. There has been much debate regarding best management, and there have been several algorithms published about the appropriate sequence of events (37-39). Initial diagnosis starts with physical exam and a pelvic radiograph. For the classic “open book” pelvic fracture, the first maneuver is the application of a pelvic binder. Closing down the volume of the pelvic space alone can control hemorrhage. If the patient does not become hemodynamically stable with the application of a binder and resuscitation, or if the fracture is not amenable to stabilization, one must proceed to an alternative treatment.
Patients who have isolated pelvic fractures may be a rare exception to the rule that the hypotensive trauma patient should be in the operating room. CT scan is performed to look for areas of arterial extravasation. Embolization by interventional radiology is the best option to control arterial bleeding as it is difficult to obtain surgical control deep within the pelvis (40). Rarely patients who have no blush on CT may still undergo empiric non-selective internal iliac artery embolization, although this places patients at significant risk for pelvic ischemia and deep tissue injury (41). This algorithm is limited in that angioembolization is often not immediately available, and embolization is a poor option to treat venous hemorrhage (40,42). The majority of patients with pelvic fractures bleed from a venous source (43).
Patients who present with hemodynamic instability are resuscitated with a Massive Transfusion Protocol and are further evaluated with a FAST exam. Once the patient has shown instability despite blood transfusions and has evidence of a pelvic fracture, there are multiple options available to the trauma surgeon. REBOA (as discussed above) deployed in Zone III can temporarily occlude arterial flow and slow hemorrhage enough to allow time for definitive hemorrhage control (23,34). Closing down the volume of the pelvic space with a binder can control hemorrhage. However, a binder cannot be left on for a prolonged period as it is associated with pressure ulcers and skin necrosis. It should be followed by definitive repair within 48 hours (44).
Another tool available to stop bleeding associated with pelvic fractures is preperitoneal pelvic packing (PPP). Several studies have shown that PPP is suitable when IR is not immediately available (37,45,46). It should be a consideration for all pelvic fractures with refractory hypotension. Denver Health Medical Center has published several studies on their implementation of PPP and has shown mortality improvement from 40% to 20% when used appropriately (41,47).
The American College of Surgeons offers the Advanced Surgical Skills for Exposure in Trauma (ASSET) course which includes PPP (48). This technique is performed by making a 6–8 cm midline incision from the pubic symphysis cephalad and dividing the midline fascia (49). Prior to entering the peritoneal cavity, there is a potential space between the fascia and peritoneum which is the preperitoneal space. The hematoma from a pelvic fracture often dissects this space down to the presacral region, making packing easy. Three laparotomy pads are placed on each side of the bladder deep within the preperitoneal space, and the wound is temporarily closed with pelvic packing left in place. The patient should return to the OR within 48 hours for pelvic packing removal (47). If the pelvis shows continued signs of bleeding, the pelvis can be repacked, but repeated procedures increase the risk of infection.
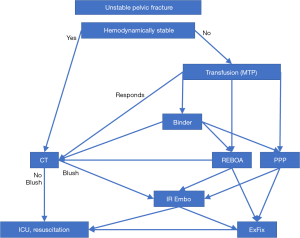
There is no consensus as to the optimal treatment algorithm for patients presenting with hemorrhage from severe pelvic fracture. Despite the strong evidence supporting use of PPP, it has not been widely accepted as standard of care. A recent multicenter review (38) of all patients presenting to 11 different level 1 trauma centers showed that of 1,339 patients presenting with pelvic fractures and hemorrhagic shock, only 35 (2.6%) were treated with preperitoneal pelvic packing. Fifteen of these patients were treated with additional interventions for hemorrhage control, including angioembolization. Angiography was performed in 9.6% of the total patients to diagnose a pelvic source of bleeding, with about half showing signs of contrast extravasation, and angioembolization was performed in 5.9% of the total. A pelvic binder was placed in 141 (10.5%) of the total. REBOA was utilized for hemorrhage control in only 6 patients at a single center. As there is no widespread acceptance of superiority of any of these choices for hemorrhage control (availability of resources varies across institutions), we propose the algorithm shown in Figure 1. Our proposed algorithm adds REBOA and is grounded on the concepts put forth in the guidelines developed by Easter Association for the Surgery of Trauma (50) and Western Trauma Association (51).
Damage control laparotomy
Ogilvie first described leaving the abdomen open at the end of a surgery as a technique to manage exsanguination and intraabdominal sepsis around World War II (52). In the 1980s, Stone et al. showed outcomes were improved when hemorrhage and contamination were controlled, the abdomen was left open, and definitive repairs were delayed until the patient could withstand the stress of surgery (53). “Damage Control Laparotomy” began to be widely used after a 1993 paper by Rotondo and colleagues identifying the major indications as the lethal triad of hypothermia, acidosis, and coagulopathy in the setting of visceral and vascular injuries (54). Since that time damage control laparotomy has slowly gained indications, now encompassing hundreds, almost all without supporting research. Surgeons may decide not to complete the fascial closure based on potential abdominal compartment syndrome, amount of blood transfused, operative duration, or multiregional injury pattern (55). Damage control laparotomy has become commonplace and is utilized in 30–40% of all trauma laparotomies (56,57).
While a useful tool for severely injured patients, DCL is not without risk for serious complications. The most dangerous complication is fistula formation, with incidence ranging from 2% to 42% (58-61). All the data on this subject is retrospective, and the patient factors and treatment practices differ greatly between studies leading to the wide range of incidence of this dreaded complication. The risk factor that affects complication rate most is duration of open abdomen and number of dressing changes (62). If a patient does develop a fistula, the mortality can be as high as 40% (63,64). The most common complication of prolonged open abdomen is chronic ventral hernia, seen in 13–80% of open abdomens (65). These are often difficult to repair and greatly impede quality of life in survivors. This has led to guidelines suggesting decreased utilization of DCL and early and aggressive attempts at closure (62).
During the same period when DCL became more widely used, resuscitation of trauma patients underwent major changes as well. As the pendulum of trauma resuscitation has swung towards balanced blood ratio resuscitation and less crystalloid, bowel edema and compartment syndrome have decreased (56,66). Decreasing use of DCL in the current landscape of trauma resuscitation has shown improved mortality from previously reported 40% to as low as 13% (60,66,67). This has led towards programs to reduce open abdomens, and stricter guidelines for its use (62,68). There will always still be a need for temporizing or damage control measures but their indications are limited (56). Taken together, these data suggest a combination of DCL, balanced resuscitation, and early aggressive efforts of diuresis and closure offer optimal outcomes.
Conclusions
Emerging techniques and technologies have advanced the field of trauma surgery to a diverse subspecialty with its own procedures and challenges. The trauma surgeon’s role has always been to orchestrate the complex interplay of the multiply injured patient and guide the patient’s progress from initial injury to final outcome. This often involves decision-making that requires facility in multiple body cavities, takes into account immediate as well as long-term consequences, and involves coordinating specialists like interventional radiology and orthopedics. REBOA, PPP and DCL are tools that are currently in a state of flux as we struggle to find their place in the armamentarium. Though the pendulum continues to swing, ultimately we hope that our continued efforts to push forward the field of trauma will lead to the appropriate application of techniques that lead to improved survival and decreased morbidity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- ATLS Subcommittee; American College of Surgeons’ Committee on Trauma; International ATLS working group. Advanced trauma life support (ATLS®): the ninth edition. J Trauma Acute Care Surg 2013;74:1363-6.
- Scalea TM, Rodriguez A, Chiu WC, et al. Focused Assessment with Sonography for Trauma (FAST): results from an international consensus conference. J Trauma 1999;46:466-72. [Crossref] [PubMed]
- Kool DR, Blickman JG. Advanced Trauma Life Support. ABCDE from a radiological point of view. Emerg Radiol 2007;14:135-41. [Crossref] [PubMed]
- American Institute of Ultrasound in Medicine, American College of Emergency Physicians. AIUM practice guideline for the performance of the focused assessment with sonography for trauma (FAST) examination. J Ultrasound Med 2014;33:2047-56. [Crossref] [PubMed]
- Ghafouri HB, Zare M, Bazrafshan A, et al. Diagnostic accuracy of emergency-performed focused assessment with sonography for trauma (FAST) in blunt abdominal trauma. Electron Physician 2016;8:2950-3. [Crossref] [PubMed]
- Knudtson JL, Dort JM, Helmer SD, et al. Surgeon-performed ultrasound for pneumothorax in the trauma suite. J Trauma 2004;56:527-30. [Crossref] [PubMed]
- Chen L, Zhang Z. Bedside ultrasonography for diagnosis of pneumothorax. Quant Imaging Med Surg 2015;5:618-23. [PubMed]
- Melniker LA, Leibner E, McKenney MG, et al. Randomized controlled clinical trial of point-of-care, limited ultrasonography for trauma in the emergency department: the first sonography outcomes assessment program trial. Ann Emerg Med 2006;48:227-35. [Crossref] [PubMed]
- Cowley RA. A total emergency medical system for the State of Maryland. Md State Med J 1975;24:37-45. [PubMed]
- Zhang Z, Hong Y, Liu N, et al. Diagnostic accuracy of contrast enhanced ultrasound in patients with blunt abdominal trauma presenting to the emergency department: a systematic review and meta-analysis. Sci Rep 2017;7:4446. [Crossref] [PubMed]
- Gamanagatti S, Rangarajan K, Kumar A, et al. Blunt abdominal trauma: imaging and intervention. Curr Probl Diagn Radiol 2015;44:321-36. [Crossref] [PubMed]
- Yumoto T, Kosaki Y, Yamakawa Y, et al. Occult Sources of Bleeding in Blunt Trauma: A Narrative Review. Acta Med Okayama 2017;71:363-8. [PubMed]
- Acar Y, Tezel O, Salman N, et al. 12th WINFOCUS world congress on ultrasound in emergency and critical care. Crit Ultrasound J 2016;8:12. [Crossref] [PubMed]
- Working Group AHSoO, American College of Surgeons. Committee on Trauma. Practice management guidelines for emergency department thoracotomy. Working Group, Ad Hoc Subcommittee on Outcomes, American College of Surgeons-Committee on Trauma. J Am Coll Surg 2001;193:303-9. [PubMed]
- Seamon MJ, Pathak AS, Bradley KM, et al. Emergency department thoracotomy: still useful after abdominal exsanguination? J Trauma 2008;64:1-7; discussion -8.
- Seamon MJ, Haut ER, Van Arendonk K, et al. An evidence-based approach to patient selection for emergency department thoracotomy: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2015;79:159-73; discussion -8. [Crossref] [PubMed]
- Stannard A, Eliason JL, Rasmussen TE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J Trauma 2011;71:1869-72. [Crossref] [PubMed]
- Martinelli T, Thony F, Decléty P, et al. Intra-aortic balloon occlusion to salvage patients with life-threatening hemorrhagic shocks from pelvic fractures. J Trauma 2010;68:942-8. [PubMed]
- Malina M, Veith F, Ivancev K, et al. Balloon occlusion of the aorta during endovascular repair of ruptured abdominal aortic aneurysm. J Endovasc Ther 2005;12:556-9. [Crossref] [PubMed]
- White JM, Cannon JW, Stannard A, et al. Endovascular balloon occlusion of the aorta is superior to resuscitative thoracotomy with aortic clamping in a porcine model of hemorrhagic shock. Surgery 2011;150:400-9. [Crossref] [PubMed]
- Moore LJ, Martin CD, Harvin JA, et al. Resuscitative endovascular balloon occlusion of the aorta for control of noncompressible truncal hemorrhage in the abdomen and pelvis. Am J Surg 2016;212:1222-30. [Crossref] [PubMed]
- Villamaria CY, Eliason JL, Napolitano LM, et al. Endovascular Skills for Trauma and Resuscitative Surgery (ESTARS) course: curriculum development, content validation, and program assessment. J Trauma Acute Care Surg 2014;76:929-35; discussion 35-6. [Crossref] [PubMed]
- Brenner ML, Moore LJ, DuBose JJ, et al. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg 2013;75:506-11. [Crossref] [PubMed]
- DuBose JJ. How I do it: Partial resuscitative endovascular balloon occlusion of the aorta (P-REBOA). J Trauma Acute Care Surg 2017;83:197-9. [Crossref] [PubMed]
- Manley JD, Mitchell BJ, DuBose JJ, et al. A Modern Case Series of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) in an Out-of-Hospital, Combat Casualty Care Setting. J Spec Oper Med 2017;17:1-8. [PubMed]
- Scott DJ, Eliason JL, Villamaria C, et al. A novel fluoroscopy-free, resuscitative endovascular aortic balloon occlusion system in a model of hemorrhagic shock. J Trauma Acute Care Surg 2013;75:122-8. [Crossref] [PubMed]
- Morrison JJ, Galgon RE, Jansen JO, et al. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg 2016;80:324-34. [Crossref] [PubMed]
- Avaro JP, Mardelle V, Roch A, et al. Forty-minute endovascular aortic occlusion increases survival in an experimental model of uncontrolled hemorrhagic shock caused by abdominal trauma. J Trauma 2011;71:720-5; discussion 5-6. [Crossref] [PubMed]
- Napolitano LM. Resuscitative Endovascular Balloon Occlusion of the Aorta: Indications, Outcomes, and Training. Crit Care Clin 2017;33:55-70. [Crossref] [PubMed]
- Johnson MA, Neff LP, Williams TK, et al. Partial resuscitative balloon occlusion of the aorta (P-REBOA): Clinical technique and rationale. J Trauma Acute Care Surg 2016;81:S133-7. [Crossref] [PubMed]
- DuBose JJ, Scalea TM, Brenner M, et al. The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: Data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg 2016;81:409-19. [Crossref] [PubMed]
- Moore LJ, Brenner M, Kozar RA, et al. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg 2015;79:523-30; discussion 30-2. [Crossref] [PubMed]
- Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients. J Trauma Acute Care Surg 2015;78:721-8. [Crossref] [PubMed]
- Ogura T, Lefor AT, Nakano M, et al. Nonoperative management of hemodynamically unstable abdominal trauma patients with angioembolization and resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg 2015;78:132-5. [Crossref] [PubMed]
- Allison SK, Ingraham C, Aarabi S, et al. Iatrogenic Common Iliac Artery Rupture from Resuscitative Endovascular Balloon Occlusion of the Aorta. J Vasc Interv Radiol 2017;28:619-20. [Crossref] [PubMed]
- Saito N, Matsumoto H, Yagi T, et al. Evaluation of the safety and feasibility of resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg 2015;78:897-903; discussion 4. [Crossref] [PubMed]
- Heetveld MJ, Harris I, Schlaphoff G, et al. Hemodynamically unstable pelvic fractures: recent care and new guidelines. World J Surg 2004;28:904-9. [Crossref] [PubMed]
- Costantini TW, Coimbra R, Holcomb JB, et al. Current management of hemorrhage from severe pelvic fractures: Results of an American Association for the Surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg 2016;80:717-23; discussion 23-5. [Crossref] [PubMed]
- Biffl WL, Smith WR, Moore EE, et al. Evolution of a multidisciplinary clinical pathway for the management of unstable patients with pelvic fractures. Ann Surg 2001;233:843-50. [Crossref] [PubMed]
- Karadimas EJ, Nicolson T, Kakagia DD, et al. Angiographic embolisation of pelvic ring injuries. Treatment algorithm and review of the literature. Int Orthop 2011;35:1381-90. [Crossref] [PubMed]
- Burlew CC, Moore EE, Stahel PF, et al. Preperitoneal pelvic packing reduces mortality in patients with life-threatening hemorrhage due to unstable pelvic fractures. J Trauma Acute Care Surg 2017;82:233-42. [Crossref] [PubMed]
- Gould JE, Vedantham S. The role of interventional radiology in trauma. Semin Intervent Radiol 2006;23:270-8. [Crossref] [PubMed]
- Huittinen VM, Slätis P. Postmortem angiography and dissection of the hypogastric artery in pelvic fractures. Surgery 1973;73:454-62. [PubMed]
- Giannoudis PV, Pape HC. Damage control orthopaedics in unstable pelvic ring injuries. Injury 2004;35:671-7. [Crossref] [PubMed]
- Verbeek DO, Sugrue M, Balogh Z, et al. Acute management of hemodynamically unstable pelvic trauma patients: time for a change? Multicenter review of recent practice. World J Surg 2008;32:1874-82. [Crossref] [PubMed]
- Li Q, Dong J, Yang Y, et al. Retroperitoneal packing or angioembolization for haemorrhage control of pelvic fractures--Quasi-randomized clinical trial of 56 haemodynamically unstable patients with Injury Severity Score ≥33. Injury 2016;47:395-401. [Crossref] [PubMed]
- Cothren CC, Osborn PM, Moore EE, et al. Preperitonal pelvic packing for hemodynamically unstable pelvic fractures: a paradigm shift. J Trauma 2007;62:834-9; discussion 9-42. [Crossref] [PubMed]
- Bowyer MW, Kuhls DA, Haskin D, et al. Advanced Surgical Skills for Exposure in Trauma (ASSET): the first 25 courses. J Surg Res 2013;183:553-8. [Crossref] [PubMed]
- Smith WR, Moore EE, Osborn P, et al. Retroperitoneal packing as a resuscitation technique for hemodynamically unstable patients with pelvic fractures: report of two representative cases and a description of technique. J Trauma 2005;59:1510-4. [Crossref] [PubMed]
- Cullinane DC, Schiller HJ, Zielinski MD, et al. Eastern Association for the Surgery of Trauma practice management guidelines for hemorrhage in pelvic fracture--update and systematic review. J Trauma 2011;71:1850-68. [Crossref] [PubMed]
- Tran TL, Brasel KJ, Karmy-Jones R, et al. Western Trauma Association Critical Decisions in Trauma: Management of pelvic fracture with hemodynamic instability-2016 updates. J Trauma Acute Care Surg 2016;81:1171-4. [Crossref] [PubMed]
- Ogilvie WH. The late complications of abdominal war-wounds. Lancet 1940;236:253-86. [Crossref]
- Stone HH, Strom PR, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg 1983;197:532-5. [Crossref] [PubMed]
- Rotondo MF, Schwab CW, McGonigal MD, et al. 'Damage control': an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma 1993;35:375-82; discussion 82-3. [Crossref] [PubMed]
- Roberts DJ, Bobrovitz N, Zygun DA, et al. Indications for use of damage control surgery and damage control interventions in civilian trauma patients: A scoping review. J Trauma Acute Care Surg 2015;78:1187-96. [Crossref] [PubMed]
- Jensen SD, Cotton BA. Damage control laparotomy in trauma. Br J Surg 2017;104:959-61. [Crossref] [PubMed]
- Hatch QM, Osterhout LM, Ashraf A, et al. Current use of damage-control laparotomy, closure rates, and predictors of early fascial closure at the first take-back. J Trauma 2011;70:1429-36. [Crossref] [PubMed]
- Huang YH, Li YS. Open abdomen in trauma patients: a double-edged sword. Mil Med Res 2016;3:10. [Crossref] [PubMed]
- Miller RS, Morris JA, Diaz JJ, et al. Complications after 344 damage-control open celiotomies. J Trauma 2005;59:1365-71; discussion 71-4. [Crossref] [PubMed]
- Lauerman MH, Dubose JJ, Stein DM, et al. Evolution of Fascial Closure Optimization in Damage Control Laparotomy. Am Surg 2016;82:1178-82. [PubMed]
- Barker DE, Green JM, Maxwell RA, et al. Experience with vacuum-pack temporary abdominal wound closure in 258 trauma and general and vascular surgical patients. J Am Coll Surg 2007;204:784-92; discussion 92-3. [Crossref] [PubMed]
- Chiara O, Cimbanassi S, Biffl W, et al. International consensus conference on open abdomen in trauma. J Trauma Acute Care Surg 2016;80:173-83. [Crossref] [PubMed]
- Tavusbay C, Genc H, Cin N, et al. Use of a vacuum-assisted closure system for the management of enteroatmospheric fistulae. Surg Today 2015;45:1102-11. [Crossref] [PubMed]
- Becker HP, Willms A, Schwab R. Small bowel fistulas and the open abdomen. Scand J Surg 2007;96:263-71. [Crossref] [PubMed]
- Smith BP, Adams RC, Doraiswamy VA, et al. Review of abdominal damage control and open abdomens: focus on gastrointestinal complications. J Gastrointestin Liver Dis 2010;19:425-35. [PubMed]
- Joseph B, Azim A, Zangbar B, et al. Improving mortality in trauma laparotomy through the evolution of damage control resuscitation: Analysis of 1,030 consecutive trauma laparotomies. J Trauma Acute Care Surg 2017;82:328-33. [Crossref] [PubMed]
- Higa G, Friese R, O'Keeffe T, et al. Damage control laparotomy: a vital tool once overused. J Trauma 2010;69:53-9. [Crossref] [PubMed]
- Harvin JA, Kao LS, Liang MK, et al. Decreasing the Use of Damage Control Laparotomy in Trauma: A Quality Improvement Project. J Am Coll Surg 2017;225:200-9. [Crossref] [PubMed]
Cite this article as: Cragun BN, Chung EL, Philp AS. Interventional and operative procedures in complex and poly-trauma. J Emerg Crit Care Med 2017;1:42.


