
Management of severe community acquired pneumonia in the emergency department
Introduction
“Knowing is not enough; we must apply. Willing is not enough; we must do.” by Johann Wolfgang von Goethe (1).
The world is witnessing a high and rising burden of community acquired pneumonia (CAP). (2). Severe CAP is a very serious and dangerous illness which is associated with high mortality (3,4). Despite advances in emergency and critical care, severe CAP is attended by poor clinical outcomes and a rising mortality (4,5). In contrast to severe CAP, survival from severe sepsis and shock has improved markedly in recent years, especially in more developed countries (6-8). This is most likely related to early recognition and aggressive resuscitation practices at the emergency room espoused and promoted by the surviving sepsis campaigns (9). In particular, the early goal directed therapy (EDGT) of septic shock is now so well adopted and executed by emergency and critical care teams in routine practice that recent controlled studies failed to detect any difference in clinical performance and patient outcomes between intervention and control groups (10-12). Sadly, this is not the case in severe CAP where mortality rates remain and high there is no agreement among experts and practitioners on many pivotal points regarding its diagnosis and management (13-15).
Rather than re-iterating these differences in opinions, we believe that, just like acute myocardial infarction and thrombotic strokes, severe CAP is a medical emergency and thus, should be managed as such (16,17). Finding practical solutions in a setting of factual uncertainty is at the root of clinical reasoning and medical decision making (18). Aristotle named this process of practical wisdom and decisions making “phronesis” which identifies, in a complex or ambiguous situation, the best rather than the right decision (where best equates to good and right equates to correct in the sense of true or approved) (19). Where prescriptive or optimal recommendations are not possible, applying an educated heuristic and taking the best possible or “satisficing” option, as described by Herbert Simon, may be expedient and appropriate (20-23). Thus, we will describe the management of severe CAP in the emergency department (ED) based on the best avail evidence and our own experience over the past decade in improving the acute care of this major medical emergency.
Methods
The management of severe CAP involve complex multifaceted interventions which are not easily amenable to prospectively randomised head-to-head clinical trials. Thus, a formal quantitative meta-analysis and systematic review of severe CAP management may not yield meaning or useful results. Instead, we have performed a qualitative, aggregative type review according to the process of realist synthesis (24,25). This is based primarily upon relevant publications on the management of CAP in MEDLINE in the past 10 years. A search limited to “adults +19 years”, “clinical trials”, “reviews” and “systematic review” yielded eligible 197 publications. This was supplemented by publications from our own research files (17,26-31).
The diagnosis of CAP
It is now widely recognized that errors in the diagnosis of CAP in the ED are very common (32,33). The frequency of misdiagnosing CAP varies with the reference standard and in one high quality prospective study, it was reported that, after CT examinations, more than half the patient had to be reclassified (34,35). Patients miscoded as having pneumonia but who did not actually have CAP tend to have more comorbidities, significantly fewer respiratory symptoms (fever, cough, dyspnoea, pleuritic pain), more constitutional symptoms (general deterioration, falls) and lower mortality (36). Conversely, CAP which was missed on plain radiographs but detected on CT was not any less severe (37). Bedside ultrasound might be an alternative imaging method especially in children (38). While the current diagnosis of CAP is less than optimal, yet we have no clear evidence to suggest that a more accurate diagnosis will improve patient outcomes. It is also unlikely that there will be wide application of much more expensive imaging methods like the CT scan in the near future. Nevertheless, a careful systematic search for the source of infection and its control in all septic patients in the ED is required. This is crucial for the early and effective treatment of CAP and other causes of sepsis (39).
Early recognition of severe CAP
The search for the ideal CAP severity score has generated the most research energies, volume of publications and controversy (40-44). This journey however has made little progress in the 20 years since the first CAP scores were promulgated (40,41,43,44). In recent years this process has included a concerted search for new biomarkers which have failed to improve on either clinical CAP severity scores or patient outcomes (45). Thus, we do not recommend the routine testing of biomarkers in CAP unless it is firmly linked to a structured care program directed at improving patient outcomes with a robust audit process to evaluate its effectiveness (46).
An unintended, indirect, unappreciated and yet real consequence of this uncertainty regarding which is the best CAP severity score is delay in the application of life saving treatment steps especially in the large group of patients who do not present, initially, with easily recognizable, frank and salient features of life-threatening CAP. Now, after over two decades of intense research, there is an emerging realization and consensus that perhaps no clinic score will ever be perfect and that our research efforts should be shifted towards interventions and implementation steps which will actually improve patient outcomes instead of just receiver operating curve statistics (47).
We recommend that despite its limitations, which have been extensively reviewed in the literature, the 2007 ATS/IDSA criteria for CAP severity should be the basis for identify patients as having life-threatening CAP and who need immediate resuscitation in the ED and consideration for early admission to an intensive care unit. (27,29,42,43,48). We suggest that, in addition, just as in sepsis treatment protocols, in patients with CAP, the serum lactate levels should be measured and monitored for clearance even in non-hypotensive patients as an indication for fluid resuscitation and evaluation of its effectiveness (29,49-51). In cases of uncertainty, evaluation of all available information, careful clinical judgment in consultation with colleagues, close monitoring following initiation of prompt aggressive and resuscitation bundles on a trial and error basis is necessary.
The identification of pathogens in severe CAP
There is a large body of research on pathogens and their rapid identification using advanced molecular techniques in CAP (30,52-57). However, the promise of effective pathogen directed and timely antimicrobial treatment of CAP arising from these studies have not yet being realized (58). Thus, it is more pertinent, in the current situation, to be aware of the prevalence of the resistant bacteria causing CAP using conventional methods (59). In particular, regional differences in antibiotic-resistant pathogens in patients with pneumonia should be reflected in local CAP management guidelines (60-62). We recommend that in patients with severe CAP, respiratory tract secretions and blood cultures should be routinely tested for common bacterial pathogens using conventional methods and that in countries tuberculosis (TB) is prevalent, this should include mycobacterial smear, rapid molecular tests and cultures of the sputum. Where available, it may be desirable to test for pneumococcal and legionella antigens in the urine, but this should not be the basis for a strategy of pathogen targeted selection of initial antimicrobial treatment. We do not recommend routine testing for viral respiratory pathogens but this may be indicated in some patients and situations for infection control and public health surveillance purposes.
Early antimicrobial administration
The causative organisms for respiratory infections vary with the climate change, environmental influences, human migratory factors and socio-economic factors (63-66). Hence the aetiology of severe CAP may differ in various regions globally (31). Epidemiological studies have identified the most common pathogens causing severe CAP which include Streptococcus pneumoniae, Klebsiella pneumoniae, Hemophilus influenzae, Pseudomonas aeruginosa and other gram-negative bacilli, Legionella spp, respiratory viruses such as influenza A and B and co-infections. Burkhorderia pseudomallei and Mycobacteria tuberculosis are endemic in the tropics and are associated with high mortality (26,67-70).
Major international guidelines recommend a beta- lactam (such as amoxicillin-clavulanate, ampicillin-sulbactam, cefotaxime or ceftriaxone) in combination with a macrolide (azithromycin or clarithromycin). In penicillin allergic individuals, a fluoroquinolone (such as levofloxacin) with aztreonam could be the alternative (42). Several systematic reviews and meta-analyses which studied macrolides in combination with beta-lactams in hospitalized non-critically ill CAP revealed conflicting results. Some showed a reduction in mortality with macrolide combination therapy while others did not (71-73). In critically ill patients with severe CAP, combination macrolide therapy seemed to confer a mortality benefit (70). The use of fluoroquinolone monotherapy or in combination with beta-lactams which did not show superiority to combination therapy with beta-lactams and macrolides (74,75). The use of fluoroquinolones was also shown to delay diagnosis of TB and should be avoided if TB was suspected (76).
Prompt administration of appropriate antibiotics in sepsis and septic shock had been associated with improved outcomes in observational studies (19,20,77,78). The Surviving Sepsis Campaign 2016 made a strong recommendation in administering antimicrobial treatment within an hour for sepsis and septic shock (9). The recommendation was further supported by recent studies published in SCAP that early administration of antibiotics and combination therapy are associated with improved intensive care survival (79-82). Some studies suggest that pre-hospital delays with antibiotic administration was associated with worsened survival and antibiotic therapy prior to hospital admission was associated with reduced incidence of septic shock and need for mechanical ventilation in patients with CAP (82). The efficacy of pre-hospital treatment needs further investigations as a recent trial of antibiotic administration in the ambulance did not show any benefits (83).
The patients with severe CAP who received guideline concordant antibiotics had improved mortality (84-87). This result differed from patients with health-care associated pneumonia (HCAP). This form of pneumonia was defined by the American guidelines in 2005 in patients who were from the community that had frequent healthcare contacts (88). The guidelines proposed administering broad spectrum antibiotics to target multidrug resistant pathogens. In a recent meta-analysis by Chalmers et al. showed that the HCAP definition did not accurately identify resistant pathogens or had an increased mortality compared to CAP (89). Administration of these broad-spectrum antibiotics based on these criteria did not improve outcomes (90,91). In 2011, the Europeans had declared HCAP to be a clinically irrelevant entity in European guidelines for lower respiratory tract infections and recommended to look for risk factors for multi-drug resistant pathogens (92). Recent observational studies performed in CAP revealed less than 10% of the cohort isolated multidrug resistant pathogens such as Pseudomonas aeruginosa, Methicillin-resistant Staphylococcus aureus (MRSA) and Enterobacteriaceae extended spectrum beta lactamase (PES) (93,94). Seasonal influenza remains a global health burden. Early empirical antiviral treatment may reduce mortality in hospitalised and critically ill patients as evident by the H1N1 influenza pandemic in 2009 (95,96).
Based on the current available evidence, we recommend early administration of appropriate antibiotics at the ED within an hour on identification of severe CAP in accordance to local epidemiology and resistance patterns (97). Combination therapy with macrolides would be favored instead of fluquinolones in TB endemic areas. We recommend empiric coverage to include melioidosis in areas that are endemic. We do not recommend empiric anti-pseudomonal or MRSA therapy in patients without risk factors for multi-drug resistant pathogens and in areas with low incidence of PES organisms or those with the HCAP appellation.
Hemodynamic resuscitation
In a landmark study in 2001, Rivers et al. demonstrated that EDGT reduced mortality in patients with severe sepsis and septic shock from 46.5% to 30.5% (10). The EDGT bundle consist of lactate measurements, fluid resuscitation to titrate central venous pressure 8–12 mmHg, vasopressor therapy to titrate mean arterial pressure (MAP) of 65 mmHg, red blood cell transfusion and inotropes to target the central venous oxygen (ScVO2) above 70 mmHg (29). This formed the premise of sepsis resuscitation guidelines. Observational studies from the surviving sepsis campaign database reported improved mortality with those hospitals with high adherence to sepsis bundles (98). In 2014–2015, there were three large randomized control trials (the PROCESS, ARISE and ProMISE) performed which did not show EDGT had improved outcomes compared to usual care. The patients in the EDGT arms required increased intensive care unit utilization, more vasopressors and central venous line insertions (12). These trials probably demonstrated that there was an overall improvement in sepsis resuscitation over the last decade.
The Surviving Sepsis Campaign 2016 recommended an initial fluid bolus of 30 mL/kg in sepsis induced hypotension and subsequent fluid boluses to be titrated according to hemodynamic status. This recommendation was shown to be safe in the PROCESS, ARISE and ProMISE trials as the patients recruited had at least 2 L of fluid before randomization (12). In a recent observational study Leisman et al. showed that early initiation of fluid resuscitation within 30 minutes was associated with reduction of mortality and initial volumes of 20–35mL/kg was associated with improved lactate clearance and lower risk of mechanical ventilation (99). However, the optimal fluid strategy in resuscitation is still subjected to controversy as Marik et al. demonstrated that administration of more than 5 L of fluid in the first 24 hours was associated with increased mortality regardless of severity of illness and increase hospital costs (100). This observation is supported by two randomized studies which showed that aggressive fluid loading was associated with increased risk of death (101,102).
Although these trials and guidelines are in sepsis, we recommend adopting these strategies in severe CAP as a significant proportion of the patients recruited in these trials had a pulmonary source of sepsis. We recommend an initial fluid bolus of 30 mL/kg within 30 minutes of recognition of sepsis induced hypotension and titrating subsequent fluid therapy in accordance to fluid responsiveness to avoid excessive fluid loading. Dynamic variables such as passive straight leg raises or fluid challenges with systolic pressure, pulse pressure and stroke volume variations could be utilized for assessment of fluid responsiveness. A recent systematic review by Bednarczyk et al. showed that fluid resuscitation with dynamic assessment of fluid responsiveness was associated with reduction in mortality, intensive care unit (ICU) length of stay and mechanical ventilation (103). Vasopressors could be initiated early to an MAP of 65 mmHg for patients with septic shock in patients who remain persistently hypotensive and no longer fluid responsive (104). Early lactate clearance was associated with improved mortality and could be used to guide resuscitation (105,106).
The management of acute respiratory failure
Acute respiratory failure and acute respiratory distress syndrome (ARDS) are major complications and causes of mortality in severe CAP (107,108). Acute respiratory failure or ARDS from severe CAP are major indications for prompt tracheal intubation and invasive mechanical ventilation. Early detection and stratification of respiratory failure and ARDS in pneumonia is based on the PaO2/FiO2 ratio (42). This requires serial sampling of arterial blood gas which is not convenient in most busy EDs. However, continuous, cheap, painless and non-invasive monitoring of oxygenation by pulse oximetry is routine practice in all acute medical settings. Thus, early and non-invasive detection and monitoring of ARDS can be perform efficiently by using the SpO2/FiO2 ratio (109-111). Because the imputation of PaO2/FiO2 from SpO2/FiO2 is non-linear, a decision needs to be made on pragmatic action points (112,113). In this regards an SpO2/FiO2 ratio of 235 which corresponds to an SpO2/FiO2 ratio of 200 would define the impending risk of ARDS (114). Alternatively, simple rule of thumb would be the need to increase the FiO2 of 0.4 to achieve an SpO2 of 100% (corresponding to an SpO2/FiO2 ratio of below 250). This could be translated into a simple practical rule of thumb that not patient with CAP should be admitted to a general medical ward on an FiO2 of ≥0.4.
Some patients with respiratory failure from CAP may benefit from a trial of non-invasive ventilation (NIV) (115,116). But the clinical evidence for this practice, once acute exacerbations of chronic obstructive pulmonary disease are excluded, is not robust and thus, it is not recommended in the current official ERS/ATS clinical practice guidelines on NIV for acute respiratory failure (117). Similarly, in severe CAP, delivery of oxygen via high flow nasal cannulae (HFNC) may be better tolerated but gives no clear advantages to either O2 or NIV (118-120). Moreover, just as in NIV, failure of HFNC delays tracheal intubation, is associated with sudden cardiovascular arrest and increased mortality (121). So, we suggest that in cases of uncertainty it might be safer to perform elective intubation and invasive ventilation rather than trials of either NIV or HFNC. In the latter situation, close monitoring and clear-cut time-lines and criteria for patient response versus failure should be agreed to, explicated and practiced consistently.
The role of corticosteroids
There is emerging evidence and growing consensus that treatment with systematic corticosteroids for relative adrenal failure may be indicated in patients with severe refractory septic shock (122). By contrast, despite some advocates for this treatment also in severe CAP, it is uncertain if patients with CAP who are not in shock will benefit (123-125). Corticosteroid treatment may suppress inflammatory cascades and promote transient improvements in clinical and radiological signs in patients who present with severe CAP and a strong inflammatory response (126). However, while this treatment may reduce mortality in severe CAP, it is associated with an increased risk for CAP-related rehospitalisation and hyperglycaemia (127,128). Thus, we recommend that systematic corticosteroids should be used only in patients with severe CAP who are also in refractory septic shock. Converse, in patients with severe CAP who are not also in septic shock, the benefits of steroid treatment is more equivocal and risk versus benefits of treatment should be evaluated carefully in every case (67,129).
On implementing and sustaining change
Because of its complexity and the lack of robust consensus on pivotal practice points, there is a paucity of randomized controlled trials on the management of CAP (130-132). There is also a lack of awareness among acute care physicians that severe CAP should be managed as a medical emergency with time sensitive action sequences. This is in sharp contrast with the situation in acute myocardial infarction which has similar mortality rates as (133,134). In myocardial infarction, the door-to balloon time is actively tracked, monitored, and reported as performance indicators against an established standard of practice with a view to further improvement (135). Cardiology and emergency medicine teams’ co-ordinate and work keenly together to improve their performed on this quality indicator (136).
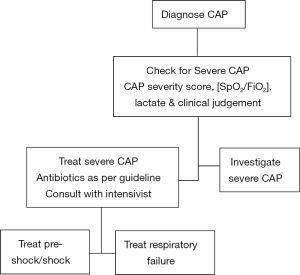
We recommend that severe CAP in the ED should be managed with an approach similar to that for severe sepsis and myocardial infarction (17,137). Timely execution of care bundles to identify severe CAP, administer initial antimicrobials, treat acute respiratory failure and septic shock are critical interventions (Figure 1) (17,29,132,138). Adept and detailed design of management programs and sustained efforts to ensure adherence to such interventions are required to translate knowledge into right, timely and effective actions at the beside (83). Monitoring appropriate metrics of health care team performance for objective, data-based, peer to peer feedback to improve adherence and accountability are key elements necessary for success and its maintenance over time (15,38). This process is similar to the implementation of severe sepsis bundles (104,137). In the case of severe CAP, in addition, we recommend careful monitoring, review, audit and feedback of all delayed admissions to the ICU (28,139). This should also include patients who died from CAP after admission to the general wards from the ED who did not enter the ICU but also did not have any prior care limitation orders.
Limitations
The recommendations and suggestion in this review are not prescriptive since they are not based on a systematic, structured, formal, hierarchical meta-analysis of the clinical evidence. They are expedient solutions based on a heuristic interpretation of the best available evidence and our own experience in managing severe CAP over the past decade. At the very least it would serve as controversial and thus, trigger points for clinicians and researchers to re-examine our preconceptions and practices in this important area.
Conclusions
We have described the management of severe CAP in the ED following a qualitative, aggregative type review of the clinical evidence. We recommend the implementation of early triage processes, prompt antimicrobial treatment and resuscitation bundles for shock and respiratory failure in the management of severe CAP in the ED. We also made suggestions for sustaining and improving upon such an enterprise. We urge acute care physicians to not wait for further evidence to support optimal care of severe CAP but to take action now to save lives and avert complications and morbidities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Goethe JW, Saunders TB. The maxims and reflections of Goethe. New York, London: Macmillan and Co., 1906:260.
- Quan TP, Fawcett NJ, Wrightson JM, et al. Increasing burden of community-acquired pneumonia leading to hospitalisation, 1998-2014. Thorax 2016;71:535-42. [Crossref] [PubMed]
- Cavallazzi R, Wiemken T, Arnold FW, et al. Outcomes in patients with community-acquired pneumonia admitted to the intensive care unit. Respir Med 2015;109:743-50. [Crossref] [PubMed]
- Lenz H, Norby GO, Dahl V, et al. Five-year mortality in patients treated for severe community-acquired pneumonia - a retrospective study. Acta Anaesthesiol Scand 2017;61:418-26. [Crossref] [PubMed]
- Rothberg MB, Pekow PS, Priya A, et al. Variation in diagnostic coding of patients with pneumonia and its association with hospital risk-standardized mortality rates: a cross-sectional analysis. Ann Intern Med 2014;160:380-8. [Crossref] [PubMed]
- Suarez De La Rica A, Gilsanz F, Maseda E. Epidemiologic trends of sepsis in western countries. Ann Transl Med 2016;4:325. [Crossref] [PubMed]
- Sánchez B, Ferrer R, Suarez D, et al. Declining mortality due to severe sepsis and septic shock in Spanish intensive care units: A two-cohort study in 2005 and 2011. Med Intensiva 2017;41:28-37. [Crossref] [PubMed]
- Elfeky S, Golabi P, Otgonsuren M, et al. The epidemiologic characteristics, temporal trends, predictors of death, and discharge disposition in patients with a diagnosis of sepsis: A cross-sectional retrospective cohort study. J Crit Care 2017;39:48-55. [Crossref] [PubMed]
- Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004;32:858-73. [Crossref] [PubMed]
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. [Crossref] [PubMed]
- Nguyen HB, Jaehne AK, Jayaprakash N, et al. Early goal-directed therapy in severe sepsis and septic shock: insights and comparisons to ProCESS, ProMISe, and ARISE. Crit Care 2016;20:160. [Crossref] [PubMed]
- Angus DC, Barnato AE, Bell D, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med 2015;41:1549-60. [Crossref] [PubMed]
- Wunderink RG, Waterer G. Advances in the causes and management of community acquired pneumonia in adults. BMJ 2017;358:j2471. [Crossref] [PubMed]
- Chalmers J, Campling J, Ellsbury G, et al. Community-acquired pneumonia in the United Kingdom: a call to action. Pneumonia (Nathan) 2017;9:15. [Crossref] [PubMed]
- Hadfield J, Bennett L. Determining best outcomes from community-acquired pneumonia and how to achieve them. Respirology 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Ewig S, Torres A. Community-acquired pneumonia as an emergency: time for an aggressive intervention to lower mortality. Eur Respir J 2011;38:253-60. [Crossref] [PubMed]
- Phua J, Dean NC, Guo Q, et al. Severe community-acquired pneumonia: timely management measures in the first 24 hours. Crit Care 2016;20:237. [Crossref] [PubMed]
- Montgomery K. How doctors think: clinical judgement and the practice of medicine. New York: Oxford University Press, 2006:246.
- Tyreman S. Promoting critical thinking in health care: phronesis and criticality. Med Health Care Philos 2000;3:117-24. [Crossref] [PubMed]
- Leahey TH, Herbert A. Simon: Nobel Prize in Economic Sciences, 1978. Am Psychol 2003;58:753-5. [Crossref] [PubMed]
- Chase VM, Hertwig R, Gigerenzer G. Visions of rationality. Trends Cogn Sci 1998;2:206-14. [Crossref] [PubMed]
- Daston L. Simon and the Sirens: A Commentary. Isis 2015;106:669-76. [Crossref] [PubMed]
- Gigerenzer G. Why Heuristics Work. Perspect Psychol Sci 2008;3:20-9. [Crossref] [PubMed]
- Gough D, Thomas J, Oliver S. Clarifying differences between review designs and methods. Syst Rev 2012;1:28. [Crossref] [PubMed]
- Wong G, Greenhalgh T, Westhorp G, et al. RAMESES publication standards: realist syntheses. BMC Med 2013;11:21. [Crossref] [PubMed]
- Lee KH, Hui KP, Tan WC, et al. Severe community-acquired pneumonia in Singapore. Singapore Med J 1996;37:374-7. [PubMed]
- Phua J, See KC, Chan YH, et al. Validation and clinical implications of the IDSA/ATS minor criteria for severe community-acquired pneumonia. Thorax 2009;64:598-603. [Crossref] [PubMed]
- Phua J, Ngerng WJ, Lim TK. The impact of a delay in intensive care unit admission for community-acquired pneumonia. Eur Respir J 2010;36:826-33. [Crossref] [PubMed]
- Lim HF, Phua J, Mukhopadhyay A, et al. IDSA/ATS minor criteria aid pre-intensive care unit resuscitation in severe community-acquired pneumonia. Eur Respir J 2014;43:852-62. [Crossref] [PubMed]
- Siow WT, Koay ES, Lee CK, et al. The Use of Polymerase Chain Reaction Amplification for the Detection of Viruses and Bacteria in Severe Community-Acquired Pneumonia. Respiration 2016;92:286-94. [Crossref] [PubMed]
- Lim TK, Siow WT. Pneumonia in the tropics. Respirology 2018;23:28-35. [Crossref] [PubMed]
- Chandra A, Nicks B, Maniago E, et al. A multicenter analysis of the ED diagnosis of pneumonia. Am J Emerg Med 2010;28:862-5. [Crossref] [PubMed]
- Sikka R, Tommaso LH, Kaucky C, et al. Diagnosis of pneumonia in the ED has poor accuracy despite diagnostic uncertainty. Am J Emerg Med 2012;30:881-5. [Crossref] [PubMed]
- Self WH, Courtney DM, McNaughton CD, et al. High discordance of chest x-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am J Emerg Med 2013;31:401-5. [Crossref] [PubMed]
- Claessens YE, Debray MP, Tubach F, et al. Early Chest Computed Tomography Scan to Assist Diagnosis and Guide Treatment Decision for Suspected Community-acquired Pneumonia. Am J Respir Crit Care Med 2015;192:974-82. [Crossref] [PubMed]
- Daniel P, Bewick T, Welham S, et al. British Thoracic S. Adults miscoded and misdiagnosed as having pneumonia: results from the British Thoracic Society pneumonia audit. Thorax 2017;72:376-9. [Crossref] [PubMed]
- Upchurch CP, Grijalva CG, Wunderink RG, et al. Community-Acquired Pneumonia Visualized on CT Scans but Not Chest Radiographs: Pathogens, Severity, and Clinical Outcomes. Chest 2017. [Epub ahead of print].
- Xin H, Li J, Hu HY. Is Lung Ultrasound Useful for Diagnosing Pneumonia in Children?: A Meta-Analysis and Systematic Review. Ultrasound Q 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Uittenbogaard AJ, de Deckere ER, Sandel MH, et al. Impact of the diagnostic process on the accuracy of source identification and time to antibiotics in septic emergency department patients. Eur J Emerg Med 2014;21:212-9. [Crossref] [PubMed]
- Neill AM, Martin IR, Weir R, et al. Community acquired pneumonia: aetiology and usefulness of severity criteria on admission. Thorax 1996;51:1010-6. [Crossref] [PubMed]
- Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997;336:243-50. [Crossref] [PubMed]
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27-72. [Crossref] [PubMed]
- Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care 2012;16:R141. [Crossref] [PubMed]
- Ranzani OT, Prina E, Menendez R, et al. New Sepsis Definition (Sepsis-3) and Community-acquired Pneumonia Mortality: A Validation and Clinical Decision-making Study. Am J Respir Crit Care Med 2017;196:1287-97. [Crossref] [PubMed]
- Viasus D, Del Rio-Pertuz G, Simonetti AF, et al. Biomarkers for predicting short-term mortality in community-acquired pneumonia: A systematic review and meta-analysis. J Infect 2016;72:273-82. [Crossref] [PubMed]
- Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018;18:95-107. [PubMed]
- Waterer G. Severity Scores and Community-acquired Pneumonia: Time to Move Forward. Am J Respir Crit Care Med 2017;196:1236-8. [Crossref] [PubMed]
- Chalmers JD, Taylor JK, Mandal P, et al. Validation of the Infectious Diseases Society of America/American Thoratic Society minor criteria for intensive care unit admission in community-acquired pneumonia patients without major criteria or contraindications to intensive care unit care. Clin Infect Dis 2011;53:503-11. [Crossref] [PubMed]
- Leisman DE, Zemmel D'Amore JA, Gribben JL, et al. Early sepsis bundle compliance for non-hypotensive patients with intermediate versus severe hyperlactemia. Am J Emerg Med 2017;35:811-8. [Crossref] [PubMed]
- Liu VX, Morehouse JW, Marelich GP, et al. Multicenter Implementation of a Treatment Bundle for Patients with Sepsis and Intermediate Lactate Values. Am J Respir Crit Care Med 2016;193:1264-70. [Crossref] [PubMed]
- Haas SA, Lange T, Saugel B, et al. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med 2016;42:202-10. [Crossref] [PubMed]
- Smith SB, Ruhnke GW, Weiss CH, et al. Trends in pathogens among patients hospitalized for pneumonia from 1993 to 2011. JAMA Intern Med 2014;174:1837-9. [Crossref] [PubMed]
- GBD 2015 LRI Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 2017;17:1133-61. [Crossref] [PubMed]
- Tan D, Fu Y, Xu J, et al. Severe adenovirus community-acquired pneumonia in immunocompetent adults: chest radiographic and CT findings. J Thorac Dis 2016;8:848-54. [Crossref] [PubMed]
- Tan D, Zhu H, Fu Y, et al. Severe Community-Acquired Pneumonia Caused by Human Adenovirus in Immunocompetent Adults: A Multicenter Case Series. PLoS One 2016;11:e0151199. [Crossref] [PubMed]
- Gadsby NJ, Russell CD, McHugh MP, et al. Comprehensive Molecular Testing for Respiratory Pathogens in Community-Acquired Pneumonia. Clin Infect Dis 2016;62:817-23. [Crossref] [PubMed]
- Huang G, Huang Q, Xie L, et al. A rapid, low-cost, and microfluidic chip-based system for parallel identification of multiple pathogens related to clinical pneumonia. Sci Rep 2017;7:6441. [Crossref] [PubMed]
- van der Eerden MM, Vlaspolder F, de Graaff CS, et al. Comparison between pathogen directed antibiotic treatment and empirical broad spectrum antibiotic treatment in patients with community acquired pneumonia: a prospective randomised study. Thorax 2005;60:672-8. [Crossref] [PubMed]
- Torres A, Cilloniz C, Ferrer M, et al. Bacteraemia and antibiotic-resistant pathogens in community acquired pneumonia: risk and prognosis. Eur Respir J 2015;45:1353-63. [Crossref] [PubMed]
- Shindo Y, Hasegawa Y. Regional differences in antibiotic-resistant pathogens in patients with pneumonia: Implications for clinicians. Respirology 2017;22:1536-46. [Crossref] [PubMed]
- Wiemken T, Peyrani P, Bryant K, et al. Incidence of respiratory viruses in patients with community-acquired pneumonia admitted to the intensive care unit: results from the Severe Influenza Pneumonia Surveillance (SIPS) project. Eur J Clin Microbiol Infect Dis 2013;32:705-10. [Crossref] [PubMed]
- Voiriot G, Visseaux B, Cohen J, et al. Viral-bacterial coinfection affects the presentation and alters the prognosis of severe community-acquired pneumonia. Crit Care 2016;20:375. [Crossref] [PubMed]
- Cillóniz C, Ewig S, Ferrer M, et al. Community-acquired polymicrobial pneumonia in the intensive care unit: aetiology and prognosis. Crit Care 2011;15:R209. [Crossref] [PubMed]
- Mirsaeidi M, Motahari H, Taghizadeh Khamesi M, et al. Climate Change and Respiratory Infections. Ann Am Thorac Soc 2016;13:1223-30. [Crossref] [PubMed]
- Cillóniz C, Ewig S, Polverino E, et al. Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax 2011;66:340-6. [Crossref] [PubMed]
- Spoorenberg SM, Bos WJ, Heijligenberg R, et al. Microbial aetiology, outcomes, and costs of hospitalisation for community-acquired pneumonia; an observational analysis. BMC Infect Dis 2014;14:335. [Crossref] [PubMed]
- Meduri GU, Bridges L, Shih MC, et al. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients' data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med 2016;42:829-40. [Crossref] [PubMed]
- Rello J, Rodriguez R, Jubert P, et al. Severe community-acquired pneumonia in the elderly: epidemiology and prognosis. Study Group for Severe Community-Acquired Pneumonia. Clin Infect Dis 1996;23:723-8. [Crossref] [PubMed]
- Tan YK, Khoo KL, Chin SP, et al. Aetiology and outcome of severe community-acquired pneumonia in Singapore. Eur Respir J 1998;12:113-5. [Crossref] [PubMed]
- Paganin F, Lilienthal F, Bourdin A, et al. Severe community-acquired pneumonia: assessment of microbial aetiology as mortality factor. Eur Respir J 2004;24:779-85. [Crossref] [PubMed]
- Nie W, Li B, Xiu Q. β-Lactam/macrolide dual therapy versus β-lactam monotherapy for the treatment of community-acquired pneumonia in adults: a systematic review and meta-analysis. J Antimicrob Chemother 2014;69:1441-6. [Crossref] [PubMed]
- Sligl WI, Asadi L, Eurich DT, et al. Macrolides and mortality in critically ill patients with community-acquired pneumonia: a systematic review and meta-analysis. Crit Care Med 2014;42:420-32. [Crossref] [PubMed]
- Garin N, Genne D, Carballo S, et al. beta-Lactam monotherapy vs beta-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med 2014;174:1894-901. [Crossref] [PubMed]
- Raz-Pasteur A, Shasha D, Paul M. Fluoroquinolones or macrolides alone versus combined with beta-lactams for adults with community-acquired pneumonia: Systematic review and meta-analysis. Int J Antimicrob Agents 2015;46:242-8. [Crossref] [PubMed]
- Skalsky K, Yahav D, Lador A, et al. Macrolides vs. quinolones for community-acquired pneumonia: meta-analysis of randomized controlled trials. Clin Microbiol Infect 2013;19:370-8. [Crossref] [PubMed]
- Chen TC, Lu PL, Lin CY, et al. Fluoroquinolones are associated with delayed treatment and resistance in tuberculosis: a systematic review and meta-analysis. Int J Infect Dis 2011;15:e211-6. [Crossref] [PubMed]
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589-96. [Crossref] [PubMed]
- Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 2014;42:1749-55. [Crossref] [PubMed]
- Rello J, Diaz E, Manez R, Sole-Violan J, et al. Improved survival among ICU-hospitalized patients with community-acquired pneumonia by unidentified organisms: a multicenter case-control study. Eur J Clin Microbiol Infect Dis 2017;36:123-30. [Crossref] [PubMed]
- Gattarello S, Borgatta B, Sole-Violan J, et al. Decrease in mortality in severe community-acquired pneumococcal pneumonia: impact of improving antibiotic strategies (2000-2013). Chest 2014;146:22-31. [Crossref] [PubMed]
- Gattarello S, Lagunes L, Vidaur L, et al. Improvement of antibiotic therapy and ICU survival in severe non-pneumococcal community-acquired pneumonia: a matched case-control study. Crit Care 2015;19:335. [Crossref] [PubMed]
- Seymour CW, Kahn JM, Martin-Gill C, et al. Delays From First Medical Contact to Antibiotic Administration for Sepsis. Crit Care Med 2017;45:759-65. [Crossref] [PubMed]
- Alam N, Oskam E, Stassen PM, et al. Prehospital antibiotics in the ambulance for sepsis: a multicentre, open label, randomised trial. Lancet Respir Med 2018;6:40-50. [PubMed]
- Frei CR, Attridge RT, Mortensen EM, et al. Guideline-concordant antibiotic use and survival among patients with community-acquired pneumonia admitted to the intensive care unit. Clin Ther 2010;32:293-9. [Crossref] [PubMed]
- Sakamoto Y, Yamauchi Y, Yasunaga H, et al. Guidelines-concordant empiric antimicrobial therapy and mortality in patients with severe community-acquired pneumonia requiring mechanical ventilation. Respir Investig 2017;55:39-44. [Crossref] [PubMed]
- Bodí M, Rodríguez A, Solé-Violán J, et al. Antibiotic prescription for community-acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis 2005;41:1709-16. [Crossref] [PubMed]
- Costantini E, Allara E, Patrucco F, et al. Adherence to guidelines for hospitalized community-acquired pneumonia over time and its impact on health outcomes and mortality. Intern Emerg Med 2016;11:929-40. [Crossref] [PubMed]
- American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. [Crossref] [PubMed]
- Chalmers JD, Rother C, Salih W, et al. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis 2014;58:330-9. [Crossref] [PubMed]
- Attridge RT, Frei CR, Pugh MJ, et al. Health care-associated pneumonia in the intensive care unit: Guideline-concordant antibiotics and outcomes. J Crit Care 2016;36:265-71. [Crossref] [PubMed]
- Haessler S, Lagu T, Lindenauer PK, et al. Treatment Trends and Outcomes in Healthcare-Associated Pneumonia. J Hosp Med 2017;12:886-91. [Crossref] [PubMed]
- Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections--full version. Clin Microbiol Infect 2011;17 Suppl 6:E1-59. [Crossref] [PubMed]
- Ishida T, Ito A, Washio Y, et al. Risk factors for drug-resistant pathogens in immunocompetent patients with pneumonia: Evaluation of PES pathogens. J Infect Chemother 2017;23:23-8. [Crossref] [PubMed]
- Prina E, Ranzani OT, Polverino E, et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc 2015;12:153-60. [Crossref] [PubMed]
- Kumar A. Early versus late oseltamivir treatment in severely ill patients with 2009 pandemic influenza A (H1N1): speed is life. J Antimicrob Chemother 2011;66:959-63. [Crossref] [PubMed]
- Smith JR, Ariano RE, Toovey S. The use of antiviral agents for the management of severe influenza. Crit Care Med 2010;38:e43-51. [Crossref] [PubMed]
- Cao B, Huang Y, She DY, et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin Respir J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015;43:3-12. [Crossref] [PubMed]
- Leisman DE, Goldman C, Doerfler ME, et al. Patterns and Outcomes Associated With Timeliness of Initial Crystalloid Resuscitation in a Prospective Sepsis and Septic Shock Cohort. Crit Care Med 2017;45:1596-606. [Crossref] [PubMed]
- Marik PE, Linde-Zwirble WT, Bittner EA, et al. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med 2017;43:625-32. [Crossref] [PubMed]
- Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med 2011;364:2483-95. [Crossref] [PubMed]
- Andrews B, Semler MW, Muchemwa L, et al. Effect of an Early Resuscitation Protocol on In-hospital Mortality Among Adults With Sepsis and Hypotension: A Randomized Clinical Trial. JAMA 2017;318:1233-40. [Crossref] [PubMed]
- Bednarczyk JM, Fridfinnson JA, Kumar A, et al. Incorporating Dynamic Assessment of Fluid Responsiveness Into Goal-Directed Therapy: A Systematic Review and Meta-Analysis. Crit Care Med 2017;45:1538-45. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Gu WJ, Zhang Z, Bakker J. Early lactate clearance-guided therapy in patients with sepsis: a meta-analysis with trial sequential analysis of randomized controlled trials. Intensive Care Med 2015;41:1862-3. [Crossref] [PubMed]
- Zhang Z, Xu X. Lactate clearance is a useful biomarker for the prediction of all-cause mortality in critically ill patients: a systematic review and meta-analysis. Crit Care Med 2014;42:2118-25. [Crossref] [PubMed]
- Mortensen EM, Coley CM, Singer DE, et al. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med 2002;162:1059-64. [Crossref] [PubMed]
- Torres A, Serra-Batlles J, Ferrer A, et al. Severe community-acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis 1991;144:312-8. [Crossref] [PubMed]
- Sanz F, Dean N, Dickerson J, et al. Accuracy of PaO2 /FiO2 calculated from SpO2 for severity assessment in ED patients with pneumonia. Respirology 2015;20:813-8. [Crossref] [PubMed]
- Rogers AJ, Liu VX. 16 Years and Counting? Time to Implement Noninvasive Screening for ARDS. Chest 2016;150:266-7. [Crossref] [PubMed]
- Chen W, Janz DR, Shaver CM, et al. Clinical Characteristics and Outcomes Are Similar in ARDS Diagnosed by Oxygen Saturation/Fio2 Ratio Compared With Pao2/Fio2 Ratio. Chest 2015;148:1477-83. [Crossref] [PubMed]
- Brown SM, Grissom CK, Moss M, et al. Nonlinear Imputation of Pao2/Fio2 From Spo2/Fio2 Among Patients With Acute Respiratory Distress Syndrome. Chest 2016;150:307-13. [Crossref] [PubMed]
- Brown SM, Duggal A, Hou PC, et al. Nonlinear Imputation of PaO2/FIO2 From SpO2/FIO2 Among Mechanically Ventilated Patients in the ICU: A Prospective, Observational Study. Crit Care Med 2017;45:1317-24. [Crossref] [PubMed]
- Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007;132:410-7. [Crossref] [PubMed]
- Xu XP, Zhang XC, Hu SL, et al. Noninvasive Ventilation in Acute Hypoxemic Nonhypercapnic Respiratory Failure: A Systematic Review and Meta-Analysis. Crit Care Med 2017;45:e727-33. [Crossref] [PubMed]
- Kondo Y, Kumasawa J, Kawaguchi A, et al. Effects of non-invasive ventilation in patients with acute respiratory failure excluding post-extubation respiratory failure, cardiogenic pulmonary edema and exacerbation of COPD: a systematic review and meta-analysis. J Anesth 2017;31:714-25. [Crossref] [PubMed]
- Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017;50:1602426. [Crossref] [PubMed]
- Leeies M, Flynn E, Turgeon AF, et al. High-flow oxygen via nasal cannulae in patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. Syst Rev 2017;6:202. [Crossref] [PubMed]
- Maitra S, Som A, Bhattacharjee S, et al. Comparison of high-flow nasal oxygen therapy with conventional oxygen therapy and noninvasive ventilation in adult patients with acute hypoxemic respiratory failure: A meta-analysis and systematic review. J Crit Care 2016;35:138-44. [Crossref] [PubMed]
- Lee CC, Mankodi D, Shaharyar S, et al. High flow nasal cannula versus conventional oxygen therapy and non-invasive ventilation in adults with acute hypoxemic respiratory failure: A systematic review. Respir Med 2016;121:100-8. [Crossref] [PubMed]
- Kang BJ, Koh Y, Lim CM, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med 2015;41:623-32. [Crossref] [PubMed]
- Annane D, Pastores SM, Rochwerg B, et al. Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med 2017;43:1751-63. [Crossref] [PubMed]
- Cheng M, Pan ZY, Yang J, et al. Corticosteroid therapy for severe community-acquired pneumonia: a meta-analysis. Respir Care 2014;59:557-63. [Crossref] [PubMed]
- Ramsey TD, Gorman SK. Corticosteroids in the treatment of severe community-acquired pneumonia. Curr Infect Dis Rep 2014;16:405. [Crossref] [PubMed]
- Wu WF, Fang Q, He GJ. Efficacy of corticosteroid treatment for severe community-acquired pneumonia: A meta-analysis. Am J Emerg Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313:677-86. [Crossref] [PubMed]
- Briel M, Spoorenberg SM, Snijders D, et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: systematic review and individual patient data meta-analysis. Clin Infect Dis 2017. [Epub ahead of print]. [PubMed]
- Stern A, Skalsky K, Avni T, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev 2017;12:CD007720. [PubMed]
- Zhang Z, Chen L, Ni H. The effectiveness of Corticosteroids on mortality in patients with acute respiratory distress syndrome or acute lung injury: a secondary analysis. Sci Rep 2015;5:17654. [Crossref] [PubMed]
- Yealy DM, Auble TE, Stone RA, et al. Effect of increasing the intensity of implementing pneumonia guidelines: a randomized, controlled trial. Ann Intern Med 2005;143:881-94. [Crossref] [PubMed]
- Filardo G, Nicewander D, Herrin J, et al. A hospital-randomized controlled trial of a formal quality improvement educational program in rural and small community Texas hospitals: one year results. Int J Qual Health Care 2009;21:225-32. [Crossref] [PubMed]
- Lim WS, Rodrigo C, Turner AM, et al. British Thoracic Society community-acquired pneumonia care bundle: results of a national implementation project. Thorax 2016;71:288-90. [Crossref] [PubMed]
- Gupta T, Patel K, Kolte D, et al. Relationship of Hospital Teaching Status with in-Hospital Outcomes for ST-Segment Elevation Myocardial Infarction. Am J Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Hayes BH, Haberling DL, Kennedy JL, et al. Burden of Pneumonia-Associated Hospitalizations - United States, 2001-2014. Chest 2017. [Epub ahead of print]. [PubMed]
- Krumholz HM, Herrin J, Miller LE, et al. Improvements in door-to-balloon time in the United States, 2005 to 2010. Circulation 2011;124:1038-45. [Crossref] [PubMed]
- Singer AJ, Shembekar A, Visram F, et al. Emergency department activation of an interventional cardiology team reduces door-to-balloon times in ST-segment-elevation myocardial infarction. Ann Emerg Med 2007;50:538-44. [Crossref] [PubMed]
- Damiani E, Donati A, Serafini G, et al. Effect of performance improvement programs on compliance with sepsis bundles and mortality: a systematic review and meta-analysis of observational studies. PLoS One 2015;10:e0125827. [Crossref] [PubMed]
- Guo Q, Li HY, Li YM, et al. Compliance with severe sepsis bundles and its effect on patient outcomes of severe community-acquired pneumonia in a limited resources country. Arch Med Sci 2014;10:970-8. [Crossref] [PubMed]
- Renaud B, Santin A, Coma E, et al. Association between timing of intensive care unit admission and outcomes for emergency department patients with community-acquired pneumonia. Crit Care Med 2009;37:2867-74. [Crossref] [PubMed]
Cite this article as: Lim TK, Chew MY. Management of severe community acquired pneumonia in the emergency department. J Emerg Crit Care Med 2018;2:2.


