
Diagnosis and therapy of sepsis
Introduction
Sepsis is a condition, which arises when the host response to infection causes organ dysfunction. Sepsis related organ dysfunction is a frequent cause of death (1) and disability in survivors (2). Sepsis is also a frequent disease. The incidence was estimated in a recent meta-analysis to 270 severe sepsis cases/100,000 person years with a mortality of 26% (3). The authors of this meta-analysis noted that population based data were only available from high-income areas (United States, Germany, Australia, Taiwan, Norway, Spain, and Sweden). Thus, authoritative epidemiological data from low- and middle-income areas representing more than 80% of the global population are missing (3). Reliable epidemiological data are essential to assess the efficiency of quality improvement programs and national treatment initiatives (4). The problem of missing population based epidemiological data has recently been mentioned also for China. It’s population of about 1.3 and 0.25 billion floating population rises severe methodological issues for obtaining such data (5).
Current data suggest that sepsis mortality has decreased over the last decades (6,7). Such reports are not without criticism. An increased vigilance for sepsis may over time cause physicians to diagnose sepsis more often also in patients with low risk of death. Thus, observations over time might be confounded by a change in patient severity rather than an improved therapy. This effect is known as the Will Rogers phenomenon or stage migration (8). In addition, sepsis mortality may differ significantly between countries and health care systems; i.e., sepsis mortality in European countries is higher than in the United States which was attributed to differences in intensive care (1,9). Sepsis mortality in China had been up to 70% in rural parts of the country where access to health care is limited. Distribution of the Surviving Sepsis Campaign (SSC)-guidelines in China was associated with a marked decrease in sepsis mortality especially in rural areas (10). The study did not differentiate between sepsis, severe sepsis, and septic shock. Thus, it is no possible to differentiate whether change in therapy, early sepsis diagnosis, or both are responsible for the decrease in mortality. The success of SSC guideline implementation in case of low guideline compliance had also been observed in Brazil (11,12). Distributing and implementing knowledge about current guidelines is a crucial part in maintaining adequate care of sepsis patients.
Recently, expert committees have changed many details in the sepsis approach. The sepsis definition has been reworked in a way that basic terminology of this condition now differs significantly from the definition implemented in clinical practice (13). In addition, the SSC has updated their recommendations for sepsis diagnosis and therapy (14). This review article summarizes the new developments in sepsis definition and in the approach for diagnosis and treatment of sepsis care.
Definition of sepsis
Sepsis-1
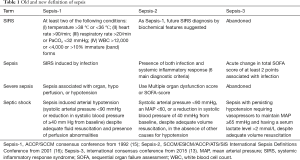
The first definition of sepsis has been published by the SCCM/ACCP consensus conference committee in 1992 (15). The committee introduced the term systemic inflammatory response syndrome (SIRS). SIRS was supposed to describe the inflammatory response not only seen in sepsis but in many other non-infectious diseases. Sepsis then was defined as systemic inflammatory response to infection. When sepsis occurred with organ dysfunction, it was called severe sepsis. Septic shock was defined as sepsis induced arterial hypotension despite adequate fluid resuscitation combined with the presence of perfusion abnormalities. This SCCM/ACCP definition of sepsis facilitated clinical sepsis research for decades by allowing the selection of comparable patient populations.
Sepsis-2
Results of a second consensus conference were published in 2001 (16). The authors mentioned sequential organ failure assessment (SOFA) as a potential definition of infection associated organ dysfunction without giving further advise for the application of such a scoring system. Septic shock was defined as persistent arterial hypotension unexplained by other causes, only. The original requirement of perfusion abnormalities mentioned in the Sepsis-1 definition disappeared. Additionally, the committee presented a possible staging system which they called PIRO. PIRO stands for predisposition, insult infection, response, and organ dysfunction. For each of the four domains, the authors presented factors possibly affecting course and outcome of sepsis. Although the PIRO-system was further investigated over the following years, PIRO did not find acceptance in clinical practice.
Sepsis-3
The previous two sepsis definitions relied on the concept of SIRS. However, this concept was soon criticized as being too unspecific for having any diagnostic value (17). Indeed, many patients admitted to the intensive care unit (ICU) fulfil two SIRS criteria without having sepsis (18). Almost half of ICU patients develop SIRS at least once during their ICU stay (19).
Sepsis-1 and Sepsis-2 were developed based on expert opinion only. To overcome the shortcomings of such an approach, the Sepsis-3 task force applied scientific methods to develop new sepsis definitions in two steps: (I) diagnostic criteria for sepsis were derived from a large database consisting of 148,907 patients with suspected infection. When determining the prognostic capability to predict in-hospital mortality, SOFA had a higher predictive validity than SIRS (20). The terms severe sepsis, SIRS, and sepsis syndrome were then eliminated from the diagnostic sepsis criteria. Sepsis now replaces the former severe sepsis while organ dysfunction is now represented by the SOFA-score (13); (II) the definition of septic shock has been derived from a meta-analysis to define a subgroup of sepsis patient with an increased risk of death more than sepsis alone. This meta-analysis confirmed the original septic shock definition combining arterial hypotension and perfusion abnormalities now measured by serum lactate (21). The diagnostic criteria of Sepsis-3 are summarized in Table 1.
Full table
Determination of the SOFA-score is time consuming and requires laboratory assessments. SOFA-score may not be promptly available in the emergency department or the normal ward. Therefore, the consensus committee introduced the quick SOFA score (qSOFA) as a screening tool. The qSOFA consists of three items: (I) altered mental status; (II) tachypnea ≥22 breaths/min; (III) arterial hypotension (systolic blood pressure ≤100 mmHg). The qSOFA is positive if at least two items are fulfilled (13). The qSOFA has a lower predictive validity of in-hospital mortality than the SOFA-score but is much easier to handle (20). The qSOFA was sometimes misinterpreted as a replacement for the now abandoned SIRS. This is not the case. In fact, the qSOFA is not part of the Sepsis-3 definition and does not diagnose sepsis. Many non-infectious conditions such as hypovolemia, heart failure and pulmonary embolism go along with more than two qSOFA-points (22). A positive qSOFA in combination with an acute infection should rather prompt the physician to consider a transfer to the ICU, to more closely look after the source of infection, and to consider fast initiation of antimicrobial therapy. This concept of identifying high risk patients by using the qSOFA has been also confirmed in other patient populations (23,24).
Sepsis-1, ACCP/SCCM consensus conference from 1992 (15); Sepsis-2, SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference from 2001 (16); Sepsis-3, international consensus conference from 2015 (13). MAP, mean arterial pressure; SIRS, systemic inflammatory response syndrome; SOFA, sequential organ failure assessment; WBC, white blood cell count.
Diagnostic workflow
According to the sepsis definition, diagnosis of sepsis consists of the diagnosis of infection and associated organ dysfunction. Thus, acute organ dysfunction must always be outruled when new infection is suspected. The SSC recommends hospital-wide sepsis performance improvement programs to facilitate early sepsis recognition (14). Outside the ICU, the qSOFA is an appropriate tool to assess the risk of the patient. If acute organ dysfunction is present, the patient is at a higher risk of death and ICU admission should be considered. Therapeutic measures such as antimicrobial therapy, hemodynamic resuscitation and breathing support have to be initiated in parallel to the diagnostic workup.
Vice versa, a new infection needs to be outruled in any case of new onset of organ dysfunction. Basic diagnostic procedures to diagnose infection in patients with suspected sepsis consist of searching for the site of infection and identifying the underlying pathogen (25). At least two sets of blood cultures should be obtained before antimicrobial therapy is started. In addition, microbiological samples need to be taken from the suspected site of infection. The site of infection needs to be confirmed. Imaging techniques such as ultrasonography, X-ray, or CT-scans are used for this purpose. Imaging studies are also important to indicate an interventional or surgical source control in addition to the antimicrobial therapy. Depending on the patient condition, it may be justified to perform a complete CT-scan of the chest and abdomen including oral and intravenous contrast (26).
Biomarkers
The SSC recommendations clearly state that antimicrobial therapy is a lifesaving therapy for sepsis patients. It is also pointed out that patients with systemic inflammation without infection should not receive antimicrobial therapy. The identification of patients with acute organ dysfunction in need of antibiotic therapy is not always obvious (14). Several biomarkers have been investigated to further guide the physician in this diagnostic approach (27). Procalcitonin is seen as the best investigated biomarker to differentiate infectious from non-infectious inflammatory states. Indeed, diagnostic accuracy for this differentiation has been shown to be fairly good (28). Other biomarkers such as interleukin-6, sTREM-1, SUPAR, presepsin etc. with less supportive studies have also been proposed to add clinical significance to the clinical assessment. However, no single biomarker so far allows a rapid and reliable discrimination between sepsis and infection without infection. Furthermore, the currently available biomarkers seem to be unreliable if viruses or fungi are the underlying pathogens of sepsis (27). In this context, the SSC-guidelines are cautious to generally recommend biomarkers for the application in patients with suspected sepsis. It is stated that biomarkers can only give an additional aid to the clinical assessment of the physician. Procalcitonin might be used to identify those patients on systemic antibiotics where infection is unlikely and antimicrobial therapy can be stopped early (14). Another application of PCT in clinical practice would be to shorten duration of antimicrobial therapy in patients with sepsis (29).
Therapy of sepsis
Anti-infectious management
Early and adequate antimicrobial therapy is the cornerstone of the anti-infective therapy in sepsis. The SSC guidelines give several recommendations regarding antimicrobial therapy in sepsis, all of which have a low quality of evidence and low grade of recommendation. Broad empirical antimicrobial therapy should be administered as soon as possible after at least two sets of blood cultures have been obtained. Timing is crucial for this patient population as mortality increases when antimicrobial therapy is delayed (30,31). The SSC recommends to apply the first dosage of antibiotics within one hour after sepsis diagnosis (14). Broad spectrum coverage is also necessary since inappropriate antimicrobial therapy is associated with a significant increase in mortality (32). In general, broad-spectrum carbapenems (i.e., meropenem, imipenem/cilastatin or doripenem) or extended-range penicillin/β-lactamase inhibitor combination (i.e., piperacillin/tazobactam or ticarcillin/clavulanate) can be recommended as first choice drugs. Third- or higher-generation cephalosporins may also be considered (14).
Patients with a very high risk of mortality such as septic shock should receive a multidrug therapy to broaden the antimicrobial spectrum. However, combination therapy should be avoided in no-shock sepsis (33,34). Whenever a combination therapy is chosen, choice of antibiotics should be reconsidered as soon as possible. Therapeutic drug monitoring is recommended for fluoroquinolones, aminoglycosides, and vancomycin. Changes of renal drug clearance and distribution volume in sepsis patients may otherwise limit clinical success rate of anti-infectious therapy and may also increase rate of adverse drug reactions (35).
Empiric antimicrobial therapy should be narrowed as soon as the underlying pathogen and its resistance pattern is identified. De-escalation strategies are safe (36) and are independently associated with a decrease in mortality (37). Patients should be screened daily for de-escalation. In general, an antimicrobial treatment duration of 7–10 days is recommended by the SSC. Such a strategy has been confirmed in patients with ventilator or hospital acquired pneumonia and intra-abdominal infections (38-40). However, it has to be pointed out that several infections such as endocarditis, invasive Candida infections, tuberculosis, S. aureus bacteremia etc. require longer durations of antimicrobial therapy. Few data are available for choosing even shorter durations of antimicrobial therapy. However, the SSC suggests that rapid clinical resolution after successful source control or in patients with urinary sepsis may allow early discontinuation of antibiotics (14). In addition, course of PCT levels may aid the physician in discontinuation of antibiotics.
Diagnostics of site of infection may reveal a need for surgical source control. The SSC recommends surgical removal of such a focus within 6–12 hours after sepsis diagnosis. Several observational studies could demonstrate a relationship between delay of surgical source control and increased mortality (31,41,42). All of these observational studies even suggest a time frame of 6 rather than 12 hours to achieve largest survival. In addition, any intravascular device should be removed if it is a suspected site of infection.
Resuscitation
Hemodynamic deterioration occurs frequently in sepsis. The strong SSC recommendations regarding hemodynamic resuscitation are summarized in Table 2. Resuscitation mainly consists of fluid resuscitation and vasopressor therapy.

Full table
Fluid resuscitation
Septic shock is usually accompanied by severe hypovolemia. Thus, fluid resuscitation is a crucial part of hemodynamic stabilization. Crystalloid solutions are recommended as first choice fluids with an initial intravenous fluid bolus of 30 mL/kg. The hemodynamic status should be evaluated regularly and fluid administration should be continued as long as hemodynamic parameters continue to improve (14). However, high cumulative fluid balance is associated with an increased risk of death (43,44). Thus, application of intravenous fluid should be given cautiously especially beyond the initial resuscitation phase (45). A conservative fluid administration is recommended in patients with acute respiratory distress syndrome (ARDS).
SSC states that balanced crystalloid solutions or saline are equivalent choices (14). No clear data are available which allow to prefer a certain crystalloid solution. However, there is some evidence that high sodium chloride load may be associated with adverse events such as hyperchloremic acidosis and renal dysfunction (46,47).
The SSC further recommends to add albumin to fluid resuscitation (weak recommendation, low quality of evidence) if large volumes of crystalloids are required (14). The administration of albumin in sepsis patients can be considered safe (48). The ALBIOS trial did not show a mortality improvement in albumin treated patients but suggested an increased survival in septic shock patients (49). Several meta-analyses also showed an improved outcome when albumin was used (50-52). However, high quality randomized controlled trials to support the SSC-recommendation regarding fluid resuscitation with albumin are missing. Application of artificial colloids in sepsis patients is not supported by current data. There is a strong recommendation against using hydroxyethyl starch (HES) solutions for fluid resuscitation in sepsis patients (14). Several studies revealed that HES is associated with acute renal failure (53) and may even increase mortality (54,55). Good evidence for using gelatine for fluid resuscitation in septic shock is missing. A meta-analysis of the available studies showed no benefit for gelatine treated patients (56).
Vasoactive medication
Arterial hypotension frequently accompanies sepsis and often persists during or after fluid resuscitation. Norepinephrine is recommended as the first-choice vasopressor to achieve a mean arterial blood pressure of at least 65 mmHg (14). Although vasopressin levels are depleted in septic shock (57), sound data for a general recommendation of vasopressin therapy are not available. An updated meta-analysis by the SSC did not show a mortality difference when comparing norepinephrine with vasopressin or terlipressin. Up to 0.03 U/min of vasopressin may be added to achieve the desired target pressure or to control norepinephrine dosage. Dopamine is only recommended as alternative to norepinephrine in selected patients. Dopamine induces arrhythmias more frequently than norepinephrine and was associated with a higher risk of death (58). SSC-guidelines recommend dobutamine if positive inotropes are required (14).
Goals of resuscitation
Originally, the early goal directed therapy (EGDT) algorithm defined the therapeutic measures during the initial resuscitation of septic shock patients (59). EGDT consisted of recommendations regarding fluid therapy, vasopressor therapy, red blood cell (RBC) transfusion, and positive inotropes. The treatment choices were based on mean arterial pressure (MAP), central venous pressure (CVP), and central venous oxygen saturation as triggers. However, three large randomized trials could not confirm a benefit of EGDT (60-62). Therefore, EGDT is no longer recommended as treatment algorithm in septic shock patients (14).
Instead of CVP, which does not adequately predict fluid response (63), physicians should use dynamic measures to guide fluid resuscitation. These measures include passive leg raising, fluid challenge, and pulse pressure/stroke volume variation in mechanically ventilated patients (14,64). Fluid resuscitation should be continued as long as hemodynamics improve. In patients with increased lactate levels, resuscitation strategy should aim to reduce serum lactate concentrations (lactate-clearance) since such an approach is associated with better outcome (65).
MAP remains an important treatment goal. Norepinephrine should be titrated to MAP of 65 mmHg. Patients with a history of arterial hypertension might require higher blood pressures (66). As in other shock states, cardiac output monitoring or echocardiography for cardiac function assessment should be considered if the patients does not respond to initial therapy (64).
Sepsis bundles
Bundles are a set of interventions which have a high likelihood of improving mortality when adequately implemented. Thus, a set of sepsis bundles had been defined in cooperation of the SSC with the Institute of Health Care Improvement (67). The bundles are an extract of core interventions from the SSC recommendations. The last major revision of the sepsis bundles specified two sets of interventions scheduled within 3 and 6 hours after time of sepsis presentation (68). Time of sepsis presentation is defined as the earliest chart annotation where all elements of sepsis definition are fulfilled. In the emergency department, time of triage sets the time of presentation. Elements of the current sepsis bundles are presented in Table 3. It has to be noted that the current sepsis bundles—as published on the webpage of the SSC—still recommend the measurement of CVP and central-venous oxygen saturation when initial resuscitation remain unresponsive. This is not in line with the current SSC-recommendation (14). The SSC bundles are currently under revision.

Full table
Other supportive care
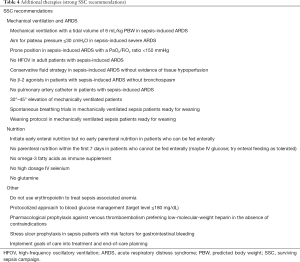
Patients with sepsis may develop any possible organ dysfunction. These patients may therefore require the whole spectrum of intensive care. Any deficite in implementing proper ICU treatments may undo the benefits on mortality won by early initiation of anti-infectious therapy and early hemodynamic resuscitation. Therefore, the SSC-guidelines make comprehensive recommendations regarding the management of these critically ill patients (14). This includes recommendations regarding the usage of blood products, anticoagulants, mechanical ventilation, sedation and analgesia, glucose control, renal replacement therapy, bicarbonate therapy, venous thromboembolismprophylaxis, stress ulcer prophylaxis, nutrition, and setting goals of care. It is beyond the scope of this review article to summarize all recommendations in detail. Strong SSC-recommendations are summarized in Table 4. Most of these recommendations which are based on high level of evidence refer to treatment of patients with ARDS and strategies for nutrition.
Full table
Several treatment strategies tried to address the systemic inflammation as one of the underlying mechanism for the development of the multiorgan dysfunction syndrome. The inflammatory host response in sepsis may be addressed by treatment with hydrocortisone, immunoglobulins, and cytokine removal techniques. High dose hydrocortisone has been shown to increase sepsis mortality. However, low dose hydrocortisone (<300 mg/day) also affects cytokine levels and compared to placebo was associated with a lower mortality in a randomized trial in septic shock patients (69). This finding was not confirmed in the large European CORTICUS trial (70). Several meta-analyses have been published showing different results depending on the studies included (71-73). In addition, it was also pointed out that less severely sick patients had been included into the CORTICUS trial compared to the study by Annane. Thus, patients of the CORTICUS trial might have been less likely to profit from hydrocortisone (74). Currently, the ADRENAL study is undertaken to clarify this issue (75). In the light of these inconclusive data, SSC-guidelines recommend 200 mg intravenous hydrocortisone only in those patients with sepsis who remain hemodynamically unstable despite adequate fluid resuscitation and vasopressor therapy (14). Hydrocortisone administration in sepsis patients without vasopressor support could not prevent progression into septic shock (76).
Immunoglobulins are used in sepsis patients for its pleiotropic effects on pathogens and host response to infection. However, the SSC guidelines recommend against the application of intravenous immunoglobulins (14) since the largest trial on this topic—the SBIT study (77)—did not show a mortality benefit when using immunoglobulins in sepsis patients. The latest meta-analysis did also not show a mortality benefit for polyclonal immunoglobulins when only studies with low risk of bias were analyzed (78). There is an ongoing debate whether immunoglobulin M-enriched polyclonal Ig (IVIgGM) is a better alternative since the mentioned meta-analysis cited seven studies showing a mortality benefit for IVIgGM (78). This was confirmed by results from a recent retrospective analysis of 100 patients treated with IVIgGM (79). However, the quality of supporting evidence for the use of IVIgGM is low. SSC-guidelines do not give a separate recommendation for IVIgGM.
The triggers of the inflammatory host response in sepsis are bacterial toxins and cytokines. Removing these substances by blood purification techniques is an intriguing concept. The SSC-guidelines do not make a recommendation regarding blood purification techniques as a clear benefit has not yet been proven (14). A meta-analysis including trials with different blood purification techniques showed a mortality benefit for patients treated with blood purification (80). This benefit was mainly driven by studies using the polymyxin B-immobilized fiber column (PMX-DHP) which removes endotoxin from the blood. Most studies showing some positive effect have been performed in Japan. However, the Japanese sepsis guidelines concluded to not recommend PMX-DHP therapy since it was questioned that removal of endotoxin alone is an effective treatment (81). Hemoadsorbtion for removal of cytokines is a newer more promising technique. Effective cytokine removal has been shown in animal models (82). However, a randomized trial with a coupled plasma filtration adsorption was stopped early because of futility (83). Further research is necessary to define the place of this concept in sepsis therapy.
Selenium is an important cofactor for the glutathione peroxidase which is part of the anti-oxidative system for the removal of oxygen free radicals. Sepsis is associated with both low selenium levels and a reduced activity of the glutathione peroxidase (84). It has been suggested that high dose selenium may improve survival of sepsis patients (85,86). However, recent large randomized trials and a subsequent meta-analysis do not support this hypothesis (87-89). Currently, the treatment of sepsis patients with high doses of selenium is not recommended. This recommendation does not outrule the nutritional supplementation of selenium in the daily recommended dosage.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author reported receiving lecture honoraria from Biosyn, Gilead, and CSL Behring and support for a clinical trial from Associates of CapeCod Inc.
References
- Levy MM, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012;12:919-24. [Crossref] [PubMed]
- Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010;304:1787-94. [Crossref] [PubMed]
- Fleischmann C, Scherag A, Adhikari NKJ, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med 2016;193:259-72. [Crossref] [PubMed]
- Finfer S, Machado FR. The Global Epidemiology of Sepsis. Does It Matter That We Know So Little? Am J Respir Crit Care Med 2016;193:228-30. [Crossref] [PubMed]
- Liao X, Du B, Lu M, et al. Current epidemiology of sepsis in mainland China. Ann Transl Med 2016;4:324. [Crossref] [PubMed]
- Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014;311:1308-16. [Crossref] [PubMed]
- Stevenson EK, Rubenstein AR, Radin GT, et al. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med 2014;42:625-31. [Crossref] [PubMed]
- Iwashyna TJ, Angus DC. Declining case fatality rates for severe sepsis: good data bring good news with ambiguous implications. JAMA 2014;311:1295-7. [Crossref] [PubMed]
- SepNet Critical Care Trials Group. Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med 2016;42:1980-9. [Crossref] [PubMed]
- Chen XC, Yang YF, Wang R, et al. Epidemiology and microbiology of sepsis in mainland China in the first decade of the 21st century. Int J Infect Dis 2015;31:9-14. [Crossref] [PubMed]
- Shiramizo SC, Marra AR, Durão MS, et al. Decreasing mortality in severe sepsis and septic shock patients by implementing a sepsis bundle in a hospital setting. PLoS One 2011;6:e26790. [Crossref] [PubMed]
- Noritomi DT, Ranzani OT, Monteiro MB, et al. Implementation of a multifaceted sepsis education program in an emerging country setting: clinical outcomes and cost-effectiveness in a long-term follow-up study. Intensive Care Med 2014;40:182-91. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 1992;101:1481-3. [Crossref] [PubMed]
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250-6. [Crossref] [PubMed]
- Vincent JL. Dear SIRS, I’m sorry to say that I don’t like you. Crit Care Med 1997;25:372-4. [Crossref] [PubMed]
- Rangel-Frausto MS, Pittet D, Costigan M, et al. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 1995;273:117-23. [Crossref] [PubMed]
- Churpek MM, Zadravecz FJ, Winslow C, et al. Incidence and Prognostic Value of the Systemic Inflammatory Response Syndrome and Organ Dysfunctions in Ward Patients. Am J Respir Crit Care Med 2015;192:958-64. [Crossref] [PubMed]
- Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:762-74. [Crossref] [PubMed]
- Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:775-87. [Crossref] [PubMed]
- Vincent JL, Martin GS, Levy MM. qSOFA does not replace SIRS in the definition of sepsis. Crit Care 2016;20:210. [Crossref] [PubMed]
- Freund Y, Lemachatti N, Krastinova E, et al. Prognostic Accuracy of Sepsis-3 Criteria for In-Hospital Mortality Among Patients With Suspected Infection Presenting to the Emergency Department. JAMA 2017;317:301-8. [Crossref] [PubMed]
- Donnelly JP, Safford MM, Shapiro NI, et al. Application of the Third International Consensus Definitions for Sepsis (Sepsis-3) Classification: a retrospective population-based cohort study. Lancet Infect Dis 2017;17:661-70. [Crossref] [PubMed]
- Bloos F. Clinical diagnosis of sepsis and the combined use of biomarkers and culture- and non-culture-based assays. Methods Mol Biol 2015;1237:247-60. [Crossref] [PubMed]
- Marshall JC, Maier RV, Jimenez M, et al. Source control in the management of severe sepsis and septic shock: an evidence-based review. Crit Care Med 2004;32:S513-26. [Crossref] [PubMed]
- Bloos F, Reinhart K. Rapid diagnosis of sepsis. Virulence 2014;5:154-60. [Crossref] [PubMed]
- Wacker C, Prkno A, Brunkhorst FM, et al. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:426-35. [Crossref] [PubMed]
- Zhang T, Wang Y, Yang Q, et al. Procalcitonin-guided antibiotic therapy in critically ill adults: a meta-analysis. BMC Infect Dis 2017;17:514. [Crossref] [PubMed]
- Ferrer R, Artigas A, Suarez D, et al. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med 2009;180:861-6. [Crossref] [PubMed]
- Bloos F, Rüddel H, Thomas-Rüddel D, et al. Effect of a multifaceted educational intervention for anti-infectious measures on sepsis mortality: a cluster randomized trial. Intensive Care Med 2017;43:1602-12. [Crossref] [PubMed]
- Kumar A, Ellis P, Arabi Y, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009;136:1237-48. [Crossref] [PubMed]
- Kumar A, Safdar N, Kethireddy S, et al. A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: a meta-analytic/meta-regression study. Crit Care Med 2010;38:1651-64. [Crossref] [PubMed]
- Kumar A, Zarychanski R, Light B, et al. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med 2010;38:1773-85. [Crossref] [PubMed]
- Roberts JA, Lipman J. Antibacterial dosing in intensive care: pharmacokinetics, degree of disease and pharmacodynamics of sepsis. Clin Pharmacokinet 2006;45:755-73. [Crossref] [PubMed]
- Guo Y, Gao W, Yang H, et al. De-escalation of empiric antibiotics in patients with severe sepsis or septic shock: A meta-analysis. Heart Lung 2016;45:454-9. [Crossref] [PubMed]
- Gutiérrez-Pizarraya A, Leone M, Garnacho-Montero J, et al. Collaborative approach of individual participant data of prospective studies of de-escalation in non-immunosuppressed critically ill patients with sepsis. Expert Rev Clin Pharmacol 2017;10:457-65. [Crossref] [PubMed]
- Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 2003;290:2588-98. [Crossref] [PubMed]
- Sawyer RG, Claridge JA, Nathens AB, et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med 2015;372:1996-2005. [Crossref] [PubMed]
- Pugh R, Grant C, Cooke RP, et al. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev 2015;7:CD007577. [PubMed]
- Azuhata T, Kinoshita K, Kawano D, et al. Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Critical Care 2014;18:R87. [Crossref] [PubMed]
- Bloos F, Thomas-Rüddel D, Rüddel H, et al. Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: a prospective observational multi-center study. Critical Care 2014;18:R42. [Crossref] [PubMed]
- Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011;39:259-65. [Crossref] [PubMed]
- Malbrain ML, Marik PE, Witters I, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther 2014;46:361-80. [Crossref] [PubMed]
- Vincent JL, De Backer D. Circulatory shock. N Engl J Med 2013;369:1726-34. [Crossref] [PubMed]
- Rochwerg B, Alhazzani W, Sindi A, et al. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med 2014;161:347-55. [Crossref] [PubMed]
- Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012;308:1566-72. [Crossref] [PubMed]
- SAFE Study Investigators. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med 2007;357:874-84. [Crossref] [PubMed]
- Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 2014;370:1412-21. [Crossref] [PubMed]
- Xu JY, Chen QH, Xie JF, et al. Comparison of the effects of albumin and crystalloid on mortality in adult patients with severe sepsis and septic shock: a meta-analysis of randomized clinical trials. Critical Care 2014;18:702. [Crossref] [PubMed]
- Jiang L, Jiang S, Zhang M, et al. Albumin versus other fluids for fluid resuscitation in patients with sepsis: a meta-analysis. PLoS One 2014;9:e114666. [Crossref] [PubMed]
- Patel A, Laffan MA, Waheed U, et al. Randomised trials of human albumin for adults with sepsis: systematic review and meta-analysis with trial sequential analysis of all-cause mortality. BMJ 2014;349:g4561. [Crossref] [PubMed]
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358:125-39. [Crossref] [PubMed]
- Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012;367:124-34. [Crossref] [PubMed]
- Haase N, Perner A, Hennings LI, et al. Hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis. BMJ 2013;346:f839. [Crossref] [PubMed]
- Moeller C, Fleischmann C, Thomas-Rueddel D, et al. How safe is gelatin? A systematic review and meta-analysis of gelatin-containing plasma expanders vs crystalloids and albumin. J Crit Care 2016;35:75-83. [Crossref] [PubMed]
- Landry DW, Levin HR, Gallant EM, et al. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation 1997;95:1122-5. [Crossref] [PubMed]
- De Backer D, Aldecoa C, Njimi H, et al. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012;40:725-30. [Crossref] [PubMed]
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. [Crossref] [PubMed]
- ARISE Investigators. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496-506. [Crossref] [PubMed]
- Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015;372:1301-11. [Crossref] [PubMed]
- ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014;370:1683-93. [Crossref] [PubMed]
- Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med 2013;41:1774-81. [Crossref] [PubMed]
- Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Med. Intensive Care Med 2014;40:1795-815. [Crossref] [PubMed]
- Jones AE, Shapiro NI, Trzeciak S, et al. Lactate Clearance vs Central Venous Oxygen Saturation as Goals of Early Sepsis Therapy A Randomized Clinical Trial. JAMA 2010;303:739-46. [Crossref] [PubMed]
- Asfar P, Meziani F, Hamel JF, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med 2014;370:1583-93. [Crossref] [PubMed]
- Levy MM, Pronovost PJ, Dellinger RP, et al. Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome. Critical Care Medicine 2004;32:S595-7. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228. [Crossref] [PubMed]
- Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002;288:862-71. [Crossref] [PubMed]
- Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008;358:111-24. [Crossref] [PubMed]
- Annane D, Bellissant E, Bollaert P-E, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA 2009;301:2362-75. [Crossref] [PubMed]
- Sligl WI, Milner DA, Sundar S, et al. Safety and efficacy of corticosteroids for the treatment of septic shock: A systematic review and meta-analysis. Clin Infect Dis 2009;49:93-101. [Crossref] [PubMed]
- Volbeda M, Wetterslev J, Gluud C, et al. Glucocorticosteroids for sepsis: systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 2015;41:1220-34. [Crossref] [PubMed]
- Mason PE, Al-Khafaji A, Milbrandt EB, et al. CORTICUS: the end of unconditional love for steroid use? Critical Care 2009;13:309. [Crossref] [PubMed]
- Venkatesh B, Myburgh J, Finfer S, et al. The ADRENAL study protocol: adjunctive corticosteroid treatment in critically ill patients with septic shock. Crit Care Resusc 2013;15:83-8. [PubMed]
- Keh D, Trips E, Marx G, et al. Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis: The HYPRESS Randomized Clinical Trial. JAMA 2016;316:1775-85. [Crossref] [PubMed]
- Werdan K, Pilz G, Bujdoso O, et al. Score-based immunoglobulin G therapy of patients with sepsis: the SBITS study. Crit Care Med 2007;35:2693-701. [PubMed]
- Alejandria MM, Lansang MA, Dans LF, et al. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst Rev 2013;273:CD001090. [PubMed]
- Giamarellos-Bourboulis EJ, Tziolos N, Routsi C, et al. Improving outcomes of severe infections by multidrug-resistant pathogens with polyclonal IgM-enriched immunoglobulins. Clin Microbiol Infect 2016;22:499-506. [Crossref] [PubMed]
- Zhou F, Peng Z, Murugan R, et al. Blood purification and mortality in sepsis: a meta-analysis of randomized trials. Crit Care Med 2013;41:2209-20. [Crossref] [PubMed]
- Oda S, Aibiki M, Ikeda T, et al. The Japanese guidelines for the management of sepsis. J Intensive Care 2014;2:55. [Crossref] [PubMed]
- Peng ZY, Carter MJ, Kellum JA. Effects of hemoadsorption on cytokine removal and short-term survival in septic rats. Critical Care Medicine 2008;36:1573-77. [Crossref] [PubMed]
- Livigni S, Bertolini G, Rossi C, et al. Efficacy of coupled plasma filtration adsorption (CPFA) in patients with septic shock: a multicenter randomised controlled clinical trial. BMJ Open 2014;4:e003536. [Crossref] [PubMed]
- Valenta J, Brodská H, Drabek T, et al. High-dose selenium substitution in sepsis: a prospective randomized clinical trial. Intensive Care Med 2011;37:808-15. [Crossref] [PubMed]
- Angstwurm MW, Engelmann L, Zimmermann T, et al. Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med 2007;35:118-26. [Crossref] [PubMed]
- Heyland DK, Dhaliwal R, Suchner U, et al. Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med 2005;31:327-37. [Crossref] [PubMed]
- Bloos F, Trips E, Nierhaus A, et al. Effect of Sodium Selenite Administration and Procalcitonin-Guided Therapy on Mortality in Patients With Severe Sepsis or Septic Shock: A Randomized Clinical Trial. JAMA Intern Med 2016;176:1266-76. [Crossref] [PubMed]
- Heyland D, Muscedere J, Wischmeyer PE, et al. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 2013;368:1489-97. [Crossref] [PubMed]
- Manzanares W, Lemieux M, Elke G, et al. High-dose intravenous selenium does not improve clinical outcomes in the critically ill: a systematic review and meta-analysis. Crit Care 2016;20:356. [Crossref] [PubMed]
Cite this article as: Bloos F. Diagnosis and therapy of sepsis. J Emerg Crit Care Med 2018;2:3.


