
Multivessel revascularization for acute myocardial infarction and cardiogenic shock: when less is more
Cardiogenic shock represents the most dreadful and the primary cause of in-hospital mortality in patients with acute myocardial infarction (AMI) (1). Cardiogenic shock (~80% of cases) occurs due to large infarction, pumps failure, infarct extension, re-infarction, or smaller infarction in preexisting left ventricular dysfunction with or without mechanical complication (2,3). Approximately 5–15% of patients with AMI are in cardiogenic shock at the time of presentation (4). Over the past 3 decades, the incidence of cardiogenic shock has been declining, a finding which has been attributed to widespread adoption of early revascularization and improvement in preventive measures. However, the prognosis of these patients remains poor. In the earlier studies such as the GUSTO-I study and the SHOCK registry, only 40% of the patients survived the hospitalization (5-8). The SHOCK trial, which randomized patients to immediate revascularization versus initial medical stabilization, showed a clear benefit of revascularization. Yet, the 30-day mortality in the patients who underwent revascularization was ~47% (5). In a recent report of the United States CathPCI registry for the years 2005–2013 showed that the in-hospital mortality has been ~30% despite increased adoption of prompt revascularization and the use of mechanical devices support as intra-aortic balloon pump (IABP) use (4), and the rates of 30 days readmissions remains high (9). Collectively, these findings suggest that we are in need for further interventions to improve the outcomes of this high-risk cohort.
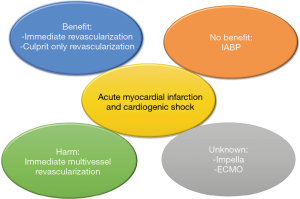
Approximately 50% of patients with STEMI exhibit one or more non-culprit lesions at the time of presentation (i.e., multivessel disease). The presence of multivessel disease has been linked to worse outcomes as compared with those with culprit-only disease (10,11). Recent randomized trials have suggested that complete revascularization of non-culprit lesions either during the index procedure or as a staged procedure is associated with improved outcomes, due to a reduction in the risk of revascularization, but with no impact on hard outcomes as death and recurrent infarction (12,13). However, these trials have excluded patients with cardiogenic shock. It would be expected that those with cardiogenic shock might drive more benefit from a complete revascularization approach. In the 2015 American College of Cardiology Foundation/American Heart Association guidelines for non-culprit vessel revascularization in STEMI patients with multivessel disease were modified from Class III indication (harm), to a Class IIb indication suggesting that it is appropriate to intervene on non-culprit lesions when cardiogenic shock persists after treatment of the culprit lesion) (14), while the 2017 European Society of Cardiology STEMI guidelines gives a class IIa recommendation for complete revascularization (15). In the SHOCK trial, the rate of multivessel PCI increased over the trial period, which perhaps suggests improved operator experiences and improved technical handling in cardiogenic shock. However, this small subset had a worse adjusted mortality compared with those who underwent culprit-only PCI (16). In a recent meta-analysis of 10 cohort studies with 6,051 patients with cardiogenic shock and multivessel disease, multivessel PCI was associated with higher early mortality (17). However, these data are driven from observational studies, which could be prone to unmeasured cofounding and ascertainment bias. Thus, a randomized trial comparing a multivessel PCI versus a cuprit-only revascularization for patients with multivessel disease would be eagerly needed.
In this context, the CULPRIT-SHOCK trial randomized 706 patients to either immediate multivessel PCI versus culprit lesion only with the option of staged revascularization of non-culprit lesions in the setting of cardiogenic shock and AMI (18). The crossover rate was relatively low (12.5% in the culprit-lesion-only PCI group and 9.4% in the multivessel PCI group). The primary end point was the composite of death or severe renal failure leading to renal-replacement therapy within 30 days after randomization. At 30 days, the composite primary end point of death or renal-replacement therapy occurred in 45.9% in the culprit-only group versus 55.4% in the multivessel PCI group [relative risk (RR) 0.83; 95% confidence interval (CI): 0.71–0.96, P=0.01], which was driven mainly by lower death in the culprit-only group (RR 0.84; 95% CI: 0.72–0.98, P=0.03). The RR of renal-replacement therapy was 0.71 (95% CI: 0.49–1.03, P=0.07). Adding renal replacement therapy as an endpoint has its relevant clinical implications since those undergoing a multivessel PCI approach are expected to receive a higher contrast volume; however, the trial showed that this does not increase the risk of renal replacement therapy. Despite the criticism that might arise from the low frequency of radial approach (i.e., <20% in both intervention groups), this trial is well conducted. The CULPRIT-SHOCK trial supports that there was a lower risk of death in patients who only had the culprit lesion treated with no difference in intensive care unit stay or duration of pressor use. This could be related to the complex interplay of various mechanistic pathways in cardiogenic shock with accelerated platelet aggregation and coagulation cascades when extra time is exerted for PCI of non-culprit lesions with further impact on ventricular function. Despite the large number of unknown deaths in the multivessel PCI group; the reported deaths were driven mainly by cerebral related deaths, despite similar stroke rates between the two groups. While complete revascularization might be of benefit in the non-shock population, this trial suggested a potential harm from this approach in the shock state. In this trial, the most common cause of death was brain injury so a plausible mechanism could be catheter manipulation.
There has been an increased interest in the use of percutaneous mechanical support devices, which has been encouraged by the poor outcomes in the cardiogenic shock population. IABP has been shown to be of no benefit in patients with cardiogenic shock who are planned for immediate revascularization (19). While other devices have been gaining interest such as continuous flow pumps (Impella) and extracorporeal membrane oxygenation (ECMO) (20). Small studies have compared IABP with new percutaneous support devices but these studies were all underpowered for hard end points (Figure 1). In the absence of data from randomized trials, the 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on mechanical support did not provide specific direction on device selection in cardiogenic shock (21). In the CULPRIT-SHOCK trial, the use of mechanical support device was nearly 30% in both arms. Impella was mainly used in the culprit lesion group while ECMO was the mainly used in the multivessel group. IABP utilization was ~25% in both arms. Despite the use of these devices, the rate of 30-day mortality in the CULPRIT-SHOCK trial (~50%) did not remarkably change from the group of patients who underwent immediate revascularization in the SHOCK trial 2 decades ago (5), these findings suggest that we are in need for further efforts to impact the outcomes of this high risk population.
In summary, the findings of the CULPRIT-SHOCK suggest that a culprit-only revascularization strategy should be the revascularization strategy of choice in patients with AMI and cardiogenic shock. This trial will impact our daily revascularization decisions, and suggests that sometimes by doing “less”, we might have “more” impact on our patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hollenberg SM. Cardiogenic shock. In: Goldman L, Ausiello D, Drazen JA. editors. Cecil textbook of medicine, 25th ed. New York: Elsevier, 2015:681-5.
- Kolte D, Khera S, Aronow WS, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc 2014;3:e000590. [Crossref] [PubMed]
- Hochman JS, Boland J, Sleeper LA, et al. Current spectrum of cardiogenic shock and effect of early revascularization on mortality. Results of an International Registry. SHOCK Registry Investigators. Circulation 1995;91:873-81. [Crossref] [PubMed]
- Wayangankar SA, Bangalore S, McCoy LA, et al. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the CathPCI registry. JACC Cardiovasc Interv 2016;9:341-51. [Crossref] [PubMed]
- Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. Shock investigators. SHould we emergently revascularize Occluded Coronaries for cardiogenic shock. N Engl J Med 1999;341:625-34. [Crossref] [PubMed]
- Goldberg RJ, Spencer FA, Gore JM, et al. Thirty-year trends (1975 to 2005) in the magnitude of management of and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation 2009;119:1211-9. [Crossref] [PubMed]
- Holmes DR Jr, Bates ER, Kleiman NS, et al. The GUSTO-I Investigators. Contemporary reperfusion therapy for cardiogenic shock: The GUSTO-I trial experience. J Am Coll Cardiol 1995;26:668-74. [Crossref] [PubMed]
- Hochman JS, Buller CE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction—etiologies, management and outcome: a report from the SHOCK Trial Registry. J Am Coll Cardiol 2000;36:1063-70. [Crossref] [PubMed]
- Mahmoud AN, Elgendy IY. Gender impact on 30-day readmissions after hospitalization with acute myocardial infarction complicated by cardiogenic shock (from the 2013 to 2014 National Readmissions Database). Am J Card 2017. [Epub ahead of print].
- Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA 2014;312:2019-27. [Crossref] [PubMed]
- Kornowski R, Mehran R, Dangas G, et al. Prognostic impact of staged versus “one-time” multivessel percutaneous intervention in acute myocardial infarction: analysis from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. J Am Coll Cardiol 2011;58:704-11. [Crossref] [PubMed]
- Elgendy IY, Wen X, Mahmoud A, et al. Complete versus culprit-only revascularization for patients with multi-vessel disease undergoing primary percutaneous coronary intervention: an updated meta-analysis of randomized trials. Catheter Cardiovasc Interv 2016;88:501-5. [Crossref] [PubMed]
- Elgendy IY, Mahmoud AN, Kumbhani DJ, et al. Complete or culprit-only revascularization for patients with multivessel coronary artery disease undergoing percutaneous coronary intervention. JACC Cardiovasc Interv 2017;10:315-24. [Crossref] [PubMed]
- Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol 2016;67:1235-50. [Crossref] [PubMed]
- Ibanez B, James S, Agewall S, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2017;39:119-177. [PubMed]
- Webb JG, Lowe AM, Sanborn TA, et al. Percutaneous coronary intervention for cardiogenic shock in the SHOCK trial. J Am Coll Cardiol 2003;42:1380-6. [Crossref] [PubMed]
- De Waha S, Jobs A, Eitel I, et al. Multivessel versus culprit lesion only percutaneous coronary intervention in cardiogenic shock complicating acute myocardial infarction: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med 2017;377:2419-32. [Crossref] [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287-96. [Crossref] [PubMed]
- Stretch R, Sauer CM, Yuh DD, et al. National trends in the utilization of short term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol 2014;64:1407-15. [Crossref] [PubMed]
- Rihal CS, Naidu SS, Givertz MM, et al. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol 2015;65:e7-26. [Crossref] [PubMed]
Cite this article as: Abuzaid A, Elgendy IY. Multivessel revascularization for acute myocardial infarction and cardiogenic shock: when less is more. J Emerg Crit Care Med 2018;2:13.


