A case of carcinomatous meningitis with occult primary malignancy
Introduction
Carcinomatous meningitis (CM) or Leptomeningeal metastasis caused by infiltration of leptomeninges by neoplastic cells. It is not uncommon and the incidence is increasing as survival rates of cancer patients improve. It has an incidence of 5% in patient suffering from solid tumours (1). Most common solid tumours associated with CM are breast carcinoma (12–35%), lung carcinoma (10–26%), malignant melanoma (5–25%), gastrointestinal tumours (4–14%), and cancers of unknown primary (1–7%) (2). Patients usually present with subtle neurological manifestations which are difficult to differentiate from those due to central nervous system (CNS) metastasis, CNS infections and adverse effects of chemotherapy. CM as a presenting feature occurs in 5–10% cases and its diagnosis in absence of and identified primary malignancy is exceptional (3). Diagnosis of CM is associated with increased morbidity and median survival rates remain dismal despite therapy.
Case presentation
A 49-year-old female with past history of diabetes mellitus type II since 4 years was admitted with complaints of progressively worsening headache since 7 days. Pain was localized to occipital area and was initially mild in intensity. Her symptoms had progressed rapidly and at the time of presentation she had a moderate to severe intensity holocranial headache. She had nausea and vomiting since 4–5 days prior to admission and also complained of photophobia with blurring of vision since 2–3 days.
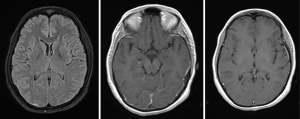
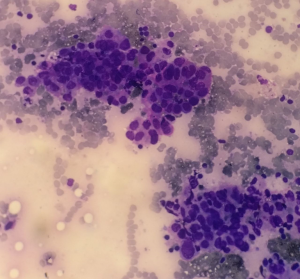
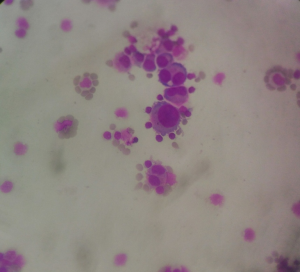
On examination the patient had signs of meningeal irritation like nuchal rigidity and positive Kernig’s and Brudzinski’s sign. There was no evidence of cranial nerve involvement, cerebellar signs, motor or sensory deficit. Her routine laboratory investigations, blood culture and urine culture were sent. Contrast MRI brain with MR venogram was done which was suggestive of increased leptomeningeal enhancement suggestive of meningitis (Figure 1). Treatment was initiated with intravenous antibiotics and supportive care. Routine cerebrospinal fluid (CSF) analysis showed 120 cells (lymphocytes 60% and polymorphs 40%), glucose 82 mg/dL and protein 71 mg/dL. Cytological analysis of CSF showed atypical cells with increased nuclear size, prominent nucleoli, irregular chromatin and moderate cytoplasm (Figure 2). PCR and culture for bacterial and viral pathogens were negative. A whole body flourodeoxy glucose (FDG) PET CT scan was advised to search for primary malignancy. FDG PET showed presence of 2 FDG avid portocaval lymph nodes. Endoscopic ultrasonography (USG) was done and needle aspiration acquired from the enlarged portocaval nodes. Cytological analysis of the aspirate was suggestive of metastatic carcinoma favouring Adenocarcinoma (Figure 3). Medical oncology consultation was taken and the patient was advised chemotherapy with gemcitabine and cisplatin.
Discussion
CM or leptomeningeal metastasis is caused by infiltration of leptomeninges with malignant cells. It has an incidence of 5% in patient suffering from solid tumours (1). Most common malignancies associated with CM are breast carcinoma (12–35%), lung carcinoma (10–26%), malignant melanoma (5–25%), gastrointestinal tumours (4–14%), and cancers of unknown primary (1–7%) (2).
Leptomeningeal metastasis is usually late features in the clinical course of malignancy. It can be the first disease manifestation in about 5% to 10% of cases. Clinical manifestations arise due to meningeal inflammation, hydrocephalus and ischemia secondary to tumour infiltration of blood vessels. It is challenging to differentiate clinically from primary CNS metastasis, CNS infection and adverse effects of chemotherapy. There is usually a background history of active or past management of malignancy.
Cancer cells reach the meningeal coverings by direct spread of tumour from contiguous CNS site, hematogenous spread through venous plexus or arteries and spread of malignant cells via perineural or periarterial spaces (3-7).
The guidelines by National Comprehensive Cancer Network (NCCN) suggest any one of the following diagnostic criteria to be sufficient to diagnose CM; CSF showing atypical cells (cells with anaplastic features); neuroradiological features consistent with CM irrespective of supportive clinical findings or clinical features suggestive of CM with a nonspecific but abnormal CSF analysis (high white blood cell count, low glucose, and elevated protein) in a known case of malignancy. MRI scan may be normal and does not entirely exclude the possibility of CM. However, in patients having a typical clinical presentation, an abnormal MRI scan alone is adequate to establish the diagnosis. CSF analysis classically reveals a high opening pressure (>200 cm of H2O), low glucose level, elevated protein level, and malignant cells on cytological examination. Elevated LDH (lactate dehydrogenase) is a nonspecific marker. In patients with positive CSF cytology the yield is 55% with single lumbar puncture and increases to 80% with a second lumbar puncture.
The treatment of CM involves systemic or intrathecal chemotherapy. Intrathecal chemotherapy most commonly consists of methotrexate and or liposomal cytarabine, often in combination with hydrocortisone (8). Radiation therapy to the leptomeninges is an effective modality in initial course as it helps to debulk tumour mass and reduces its compressive effect. Intrathecal chemotherapeutic agents may be less successful in this regard due to limited penetration (9). Systemic chemotherapeutic agents include methotrexate and cytarabine administered in high doses. The overall prognosis and disease outcome remains dismal despite the available treatments. The goal of treatment is to provide symptomatic relief for patients. Mortality results from neurological deficits with median survival ranging between 4 and 6 weeks without treatment (10).
Treatment requires the combination of surgery, radiation, and chemotherapy in most cases and can provide effective palliation with longer survival in some cases.
Conclusions
CM is a rare and late manifestation of solid tumours. CM occurring in the absence of identifiable primary malignancy is exceptional. Diagnosis is challenging due to protean CNS manifestations and low yield of neuroimaging modalities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was taken from the patient for publication of this case report and accompanying images.
References
- Kaplan JG, DeSouza TG, Farkash A, et al. Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol 1990;9:225-9. [Crossref] [PubMed]
- Gonzalez-Vitale JC, Garcia-Bunuel R. Meningeal carcinomatosis. Cancer 1976;37:2906-11. [Crossref] [PubMed]
- Balm M, Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol 1996;53:626-32. [Crossref] [PubMed]
- Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev 1999;25:103-19. [Crossref] [PubMed]
- Chamberlain MC. Radioisotope CSF flow studies in leptomeningeal metastases. J Neurooncol 1998;38:135-40. [Crossref] [PubMed]
- Boyle R, Thomas M, Adams JH. Diffuse involvement of the leptomeninges by tumour--a clinical and pathological study of 63 cases. Postgrad Med J 1980;56:149-58. [Crossref] [PubMed]
- Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer 1982;49:759-72. [Crossref] [PubMed]
- Brem SS, Bierman PJ, Brem H, et al. Central nervous system cancers. J Natl Compr Canc Netw 2011;9:352-400. [Crossref] [PubMed]
- Chang EL, Maor MH. Standard and novel radiotherapeutic approaches to neoplastic meningitis. Curr Oncol Rep 2003;5:24-8. [Crossref] [PubMed]
- Favier L, Ladoire L, Guiu B, et al. Carcinomatous Meningitis from Unknown Primary Carcinoma. Case Rep Oncol 2009;2:177-83. [Crossref] [PubMed]
Cite this article as: Trivedi T, Reddi R, Agarwal P, Arya A. A case of carcinomatous meningitis with occult primary malignancy. J Emerg Crit Care Med 2018;2:21.