
The PRESERVE trial: does it guide prevention strategies for post angiography acute kidney injury?
The PRESERVE trial, recently published in the New England Journal of Medicine, is a prospective randomized trial conducted in 53 sites including United States Veterans Administration hospitals, and sites in Malaysia, Australia and New Zealand (1). The trial randomized 5,177 patients with chronic kidney disease who were scheduled for elective coronary or peripheral artery angiography to one of four strategies for prevention of acute kidney injury (AKI). The four strategies are listed in Table 1.

Full table
The primary result of the trial was that there were no differences between any of the four strategies in either AKI incidence or 90-day adverse events (see Table 2 from publication below).
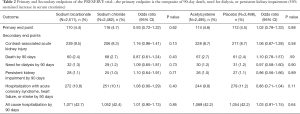
Full table
Why is this trial important?
First and foremost, it is the largest prospective randomized trial to be conducted addressing prevention of AKI post angiography. There are over 2,000,000 cardiac angiographies performed yearly in the United States alone and the incidence of AKI continues to increase (2). Currently prevention is the mainstay of therapy as there is no effective therapy for AKI once it has occurred. There have been many smaller prospective randomized trials comparing sodium bicarbonate to sodium chloride, and N-acetylcysteine to no N-acetylcysteine with variable results. Even meta-analyses have come to discordant conclusions based upon which particular studies were included in the data set. The PRESERVE trial, if representative of the patients who undergo angiography and if the protocols are consistent with how people use intravenous fluid or antioxidants for prevention, could provide a definitive answer to whether these strategies are effective.
Second, although the trial had AKI as an outcome, it was not the primary outcome. Rather the primary endpoint was a composite of 90-day events, including a sustained 50% increase in serum creatinine, the onset of dialysis dependent kidney failure, or mortality. These outcomes are more closely associated with adverse impacts on patients. AKI may be on a pathophysiologic pathway that leads to these more ‘hard’ outcomes. However, the evidence supporting a causal role for AKI is not conclusive (3,4). Equally probable is that AKI is a biomarker of the comorbidities and underlying pathophysiology of patients that leads to worsening kidney function and mortality in the future. By choosing these more ‘hard’ outcomes, the trial avoids the conundrum of interpreting AKI and its clinical role in subsequent adverse events. Additional secondary endpoints included hospitalization with acute coronary syndrome, heart failure, or stroke within 90 days; and hospitalization for any cause within 90 days. The trial found no difference in the rate of the combined primary endpoint between those who received sodium bicarbonate or sodium chloride and those who did and did not receive N-acetylcysteine in addition to either sodium bicarbonate or sodium chloride (Table 2).
While Table 2 provides the main message of the trial, it is important to look at the details of what was actually done and to assess whether or not the patients and protocols reflect general clinical practice and whether the results are generalizable to all patients undergoing angiography.
The patients
The trial enrolled only patients scheduled for an elective arteriogram. Patients with a STEMI were excluded, as were patients with unstable kidney function. Only patients with chronic kidney disease, defined as an eGFR of 15–44.9 mL/min/173 m2 or 45–59.9 mL/min/1.73 m2 with a diagnosis of diabetes, were included. This restriction to patients with chronic kidney disease is meant to ensure an adequate number of endpoints for analyses. Patients with chronic kidney disease and/or diabetes are considered ‘high risk’ in all risk models for post angiography AKI (5). The median eGFR [± IQR] was 50 mL/min/1.73 m2 [41–59] and 81% of participants had diabetes reflecting the fact that most patients had eGFR’s above 45 mL/min/m2.
The trial enrolled predominately male patients (93%) reflecting the large number of sites at VA hospitals in the US. The definition of AKI in this trial was an increase in serum creatinine of greater than 0.5 mg/dL or 25%. This is a standard definition found in the literature but is not consistent with the 2012 KDIGO definition now the current standard (6). The creatinine used to determine whether AKI occurred was obtained once between 3–5 days post angiogram and compared to a baseline creatinine obtained prior to the administration of IV fluid.
Coronary angiography was performed in 90% of the patients. Percutaneous coronary intervention (PCI) was performed in a minority (28%). The median volume of contrast was only 85 mL with 56% receiving iso-osmolal contrast and 44% low-osmolal contrast. Few patients had heart failure (38%). Most risk score models were developed in patients undergoing PCI. In those patients, more contrast is used and the increased manipulation of catheters for stent delivery increases the trauma to the aortic wall resulting in dislodgement of atheromatous material. In addition to kidney injury from contrast, those patients are susceptible to kidney injury from atheromatous emboli. The patients in PRESERVE are therefore not truly ‘high risk’ patients for AKI.
Protocol
A strong argument can be made that rather than the type of fluid administered, it is the amount of urine output that is important. The observation that less AKI occurs when more fluid is administered goes back a long way (7). More recent attempts to maximize fluid administration using hemodynamic monitoring of LVEDP (8) or CVP (9) to guide the rate of administration support the ‘more fluid is better’ hypothesis. One consequence of more fluid is that urine output is usually increased. In a seminal trial, Stevens et al. using multiple strategies for increasing urine output (fluids, dopamine, mannitol, furosemide), demonstrated that the greater the urine output the lower the rise in serum creatinine following contrast exposure (10). Indeed, patients who achieved an urine output of >3.5 L in the 24 hours following angiography had a zero incidence of AKI. The recent trials employing forced matched diuresis in which there is little change in extracellular volume but urine outputs of 300–600 mL/h for 4–6 hours after contrast exposure are induced also support the prophylactic value of a high urine output (11).
In the PRESERVE trial, urine output was not reported. The administration of IV fluid was left largely to the discretion of the clinician performing the angiogram and the total amount of fluid administered could vary considerably. The median total volume of IV fluid administered was 1,028 mL with the majority coming after angiography was performed. The median duration of IV therapy was 7 hours (2 hours pre angiography, 1 hour during angiography and 4 hours post angiography). There were no analyses of whether the volume of IV fluid administrated impacted either the primary or secondary outcomes. We are told only that the volumes were similar in all the trial groups. In many parts of the world where 1 mL/kg/h of intravenous sodium chloride for 12 hours before and after angiography is standard of care, the patients in PRESERVE would be considered under treated (based upon an average weight of 98 kg, standard of care would predict a total volume of 2,350 mL).
Pathophysiologic hypothesis
The PRESERVE trial is basically a study of antioxidant therapy for prevention of contrast associated kidney injury. There is strong evidence from animal experiments that contrast media produces mitochondrial injury leading to generation of reactive oxygen species (12). The first antioxidant to receive widespread use was N-acetylcysteine. At the time this trial was designed, the use of NAC was already in decline, largely as a result of the ACT trial published in 2013 (13). Unlike the many previous trials using NAC, the PRESERVE trial insured that the duration of NAC administration would cover the time during which injury was likely occurring. The first NAC trial for prevention of contrast associated AKI used 600 mg bid ×48 h (14). Subsequent trials used a double dose but still only for 48 hours. In PRESERVE, the double dose was given for a full 5 days ensuring that the antioxidant effect of NAC would bracket the period of injury.
Sodium bicarbonate is also an antioxidant, limited to the kidney where alkalinization of the renal medulla reduces the generation of OH− radicals generated by contrast induced mitochondrial injury of renal tubule cells (15). Again, PRESERVE took special precautions to confirm that urinary alkalinization occurred based upon urine pH measured 2–4 hours after angiography.
What doesn’t PRESERVE tell us?
PRESERVE is not a trial of more fluid versus less. There may be clues in the data regarding the incidence of AKI and the amount of fluid administered that will be the subject of later analyses. PRESERVE therefore cannot inform decisions regarding timing and amount of IV fluid to be given for protection. Furthermore, PRESERVE cannot address whether fluid administration is necessary at all. This issue was recently highlighted by the results of the AMACING trial (16).
PRESERVE is not a trial about contrast media choices. It can’t tell us the relative toxicities of isosmolal versus low osmolal contrast media or whether more contrast media is worse than less. Again, there may be hints in the data for later analysis.
PRESERVE can’t tell us whether AKI causes any of the 90-day adverse events. One can’t randomize patients to get or not get AKI and even if we technically could produce AKI on demand, it would be unethical to do so. At best, one could argue that a parallel reduction in the rate of AKI and 90-day adverse events at least supports a causal relationship between AKI and downstream events. This follows from the large trial size and the randomization procedure. Presumably (hopefully) all the variables that influence the long-term adverse events are equally distributed between the trial groups and would equally impact the incidence of those events. If a particular group then has a reduction in the incidence of AKI and a parallel decrease in the incidence of long-term events, the only variable different between the groups would be the AKI incidence. Under those circumstances, one could strongly argue that the reduction in AKI is what led to the reduction in the adverse events, the other risk variables being equal.
This is a critical issue in all trials attempting to prevent or treat AKI occurring as a result of any etiology. Observational data strongly suggests that AKI is associated with long-term cardiovascular events including congestive heart failure, progression to chronic kidney disease and dialysis, and mortality (17-19). If, as noted above, AKI is only a biomarker of risk for these adverse events but not a pathophysiologic variable causing adverse events, we should perhaps redirect our attention away from the kidney to the other risk variables associated with poor outcomes.
A final word of caution
Most trials in the domain of contrast associated AKI enroll patients with chronic kidney disease because the incidence of AKI post contrast exposure is higher in these patients. As a result of the higher incidence of AKI, smaller trials costing less money and time can be conducted. But this shouldn’t be interpreted as meaning that patients with normal kidney function are not at risk for kidney injury or the long-term adverse events mentioned above. The issue here is not whether CKD predisposes to injury: it does. One can argue that with fewer nephrons to excrete a contrast load, for example, each nephron actually has a greater exposure to the toxic effects of the contrast. Rather the often-overlooked issue is that it is both easier to diagnose AKI in patients with CKD and it takes a lesser degree of injury to reach the diagnostic threshold for AKI in patients with CKD.
It is easier because patients with CKD have lost all or most of their renal reserve that would otherwise mask the injury (20). In a healthy kidney, the loss of some nephrons through injury would be compensated by hyperfiltration in the remaining uninjured nephrons (renal reserve), leaving global glomerular filtration rate essentially unchanged despite the injury. This is less likely to happen in chronic kidney disease and the more severe the CKD, the less renal reserve is available.
Furthermore, regardless of the presence or absence of renal reserve, it takes a smaller reduction in absolute glomerular filtration rate (GFR) to reach the definition of AKI when GFR is already reduced (Table 3). For example, in a patient with a GFR of 45 mL/min or less an absolute reduction in GFR of 10 mL/min would result in a creatinine rise sufficient for a diagnosis of AKI. This same 10 mL/min loss of GFR in a patient with a baseline GFR of 50 mL/min or more would not reach the threshold for diagnosis of AKI.
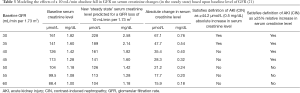
Full table
The bottom line is that what we call AKI in patients with baseline CKD may be occurring in patients without CKD but we are not recognizing it. Evidence that exposure to contrast in the absence of AKI may have long-term consequences can be found in a study by Brown and colleagues (22). They followed approximately 24,000 patients who underwent coronary angiography and compared progression to chronic kidney disease between those who developed AKI and those who didn’t as well as a 24,000 matched control group who were not exposed to contrast. Progression to a higher level of CKD was greatest in those who had AKI and least in those who were not exposed to contrast. Exposure to contrast without AKI was midway between the two.
Conclusions
In conclusion, the PRESERVE trial provides important data regarding the use of systemic and renal-limited antioxidant therapy to prevent angiography associated AKI. These strategies were not associated with benefit in males with a moderate risk of developing AKI who underwent elective, predominately diagnostic, angiography. However, the trial leaves many questions still unanswered. What is the amount and timing of intravenous fluid administration for maximum prophylaxis, the role of inducing a high urine flow rate in prophylaxis, and the type and volume of contrast media in relation to outcomes. We will probably not see another trial of this size conducted to answer these unresolved issues. We can only hope that further analyses of the PRESERVE data will provide more insights into what is the best prophylaxis in patients exposed to angiography.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Weisbord SD, Gallagher M, Jneid H, et al. Outcomes after Angiography with Sodium Bicarbonate and Acetylcysteine. N Engl J Med 2018;378:603-14. [Crossref] [PubMed]
- Brown JR, Rezaee ME, Nichols EL, et al. Incidence and In-Hospital Mortality of Acute Kidney Injury (AKI) and Dialysis-Requiring AKI (AKI-D) After Cardiac Catheterization in the National Inpatient Sample. J Am Heart Assoc 2016;5:e002739. [Crossref] [PubMed]
- Coca S. Is it AKI or nonrecovery of renal function that is important for long-term outcomes? Clin J Am Soc Nephrol 2013;8:173-6. [Crossref] [PubMed]
- Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;53:961-73. [Crossref] [PubMed]
- Allen DW, Ma B, Leung KC, et al. Risk Prediction Models for Contrast-Induced Acute Kidney Injury Accompanying Cardiac Catheterization: Systematic Review and Meta-analysis. Can J Cardiol 2017;33:724-36. [Crossref] [PubMed]
- Acute Kidney Injury Work Group. Clinical practice guideline for acute kidney injury. Kidney international Supplement 2012;2:1-138.
- Eisenberg RL, Bank WO, Hedgock MW. Renal failure after major angiography can be avoided with hydration. AJR Am J Roentgenol 1981;136:859-61. [PubMed]
- Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet 2014;383:1814-23. [Crossref] [PubMed]
- Qian G, Fu Z, Guo J, et al. Prevention of Contrast-Induced Nephropathy by Central Venous Pressure-Guided Fluid Administration in Chronic Kidney Disease and Congestive Heart Failure Patients. JACC Cardiovasc Interv 2016;9:89-96. [Crossref] [PubMed]
- Stevens MA, McCullough P, Tobin K, et al. A prospective randomized trial of prevention measures in patients at high risk for contrast nephropathy. J Am Coll Cardiol 1999;33:403-11. [Crossref] [PubMed]
- Putzu A, Boscolo Berto M, Belletti A, et al. Prevention of Contrast-Induced Acute Kidney Injury by Furosemide With Matched Hydration in Patients Undergoing Interventional Procedures: A Systematic Review and Meta-Analysis of Randomized Trials. JACC Cardiovasc Interv 2017;10:355-63. [Crossref] [PubMed]
- Heyman SN, Rosen S, Khamaisi M, et al. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol 2010;45:188-95. [Crossref] [PubMed]
- Berwanger O, Cavalcanti AB, Sousa AM, et al. Acetylcysteine for the prevention of renal outcomes in patients with diabetes mellitus undergoing coronary and peripheral vascular angiography: a substudy of the acetylcysteine for contrast-induced nephropathy trial. Circ Cardiovasc Interv 2013;6:139-45. [Crossref] [PubMed]
- Tepel M, Van Der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med 2000;343:180-4. [Crossref] [PubMed]
- Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA 2004;291:2328-34. [Crossref] [PubMed]
- Nijssen EC, Rennenberg RJ, Nelemans PJ, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomized, phase 3, controlled, open-label, non-inferiority trial. Lancet 2017;389:1312-22. [Crossref] [PubMed]
- Amdur RL, Chawla LS, Amodeo S, et al. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int 2009;76:1089-97. [Crossref] [PubMed]
- Chen SL, Zhang J, Yei F, et al. Clinical outcomes of contrast-induced nephropathy in patients undergoing percutaneous coronary intervention: a prospective, multicenter, randomized study to analyze the effect of hydration and acetylcysteine. Int J Cardiol 2008;126:407-13. [Crossref] [PubMed]
- James MT, Tonelli M, Ghali WA, et al. Renal outcomes associated with invasive versus conservative management of acute coronary syndrome: propensity matched cohort study. BMJ 2013;347:f4151. [Crossref] [PubMed]
- Ronco C, Bellomo R, Kellum J. Understanding renal functional reserve. Intensive Care Med 2017;43:917-20. [Crossref] [PubMed]
- Solomon R, Segal A. Defining acute kidney injury: what is the most appropriate metric? Nat Clin Pract Nephrol 2008;4:208-15. [Crossref] [PubMed]
- Brown JR, Solomon RJ, Robey RB, et al. Chronic Kidney Disease Progression and Cardiovascular Outcomes Following Cardiac Catheterization-A Population-Controlled Study. J Am Heart Assoc 2016;5:e003812. [Crossref] [PubMed]
Cite this article as: Solomon R. The PRESERVE trial: does it guide prevention strategies for post angiography acute kidney injury? J Emerg Crit Care Med 2018;2:29.


