
ADRENAL trial versus APROCCHSS trial: to steroid or not to steroid?
Empirical therapy with limited data
Septic shock remains a major challenge in critically ill patients with a mortality rate of 30% to 50% (1-3). The 2016 Surviving Sepsis Campaign (SSC) guidelines recommend the use of hydrocortisone to treat patients with septic shock if adequate fluid resuscitation and vasopressor therapy cannot restore hemodynamic stability (4). Although glucocorticoids have been used as an adjuvant therapy for septic shock for more than 50 years, the quality of evidence for this weak recommendation remains low (4). Two recent systemic reviews and meta-analyses have reported different conclusions on the ability of glucocorticoids to reducing 28-day mortality (5,6).
Largest randomized controlled trials to date
Two large-scale randomized controlled trials were reported in 2018 have improve the quality of evidence for using hydrocortisone to treat septic shock patients (7,8). In the ADRENAL (Adjunctive Corticosteroid Treatment in Critically Ill Patients with Septic Shock) trial (7), Venkatesh et al. reported that a continuous infusion of hydrocortisone did not decrease the 90-day mortality compared with the placebo. By contrast, in the APROCCHSS (Activated Protein C and Corticosteroids for Human Septic Shock) trial (8), Annane et al. reported that hydrocortisone plus fludrocortisone reduced 90-day all-cause mortality compared with placebo. Nevertheless, both trials indicated that faster reversal of septic shock and shorter duration of mechanical ventilation in patients receiving hydrocortisone than in those receiving placebo.
Different results, but why?
The design and outcomes of both trials are listed in Table 1. Several differences are apparent when examining the two trials. First, the APROCCHSS trial reported a 90- and 180-day survival benefit of a hydrocortisone plus fludrocortisone. Annane et al. noted that the ability of fludrocortisone to reduce mortality may be related to restore α1-adrenoceptor expression in patients with persistent vasopressor-dependent septic shock and organ failure (9), but the authors did not discuss the discrepancy between the APROCCHSS trial and their previous trial that did not indicated any survival benefit with the addition of oral fludrocortisone (10). Second, the ADRENAL trial enrolled patients from 5 countries over 4 years, whereas the APROCCHSS trial was conducted over 7 years only in France. In total, 3,713 patients completed the evaluation in the ADRENAL trial, a number three times larger than the number of patients in the APROCCHSS trial. Third, the required vasopressor dosage and timing were lower and shorter, respectively, in the ADRENAL trial than in the APROCHSS trial. This might explain higher shock severity and mortality in the APROCCHSS.

Full table
In the subgroup analysis of 90-day mortality, no survival benefit was noted in the patients with severer shock comparing to the patients with less severe shock in the ADRENAL trial. Notably, subgroup analysis of the time from shock onset to randomization in the ADRENAL trial revealed a survival benefit in the subgroup of 6–12 hours [odds ratio (OR) =0.71; 95% confidence interval (CI), 0.54–0.94]. Further investigation could explore the optimal timing of hydrocortisone use.
Patient selection makes all the difference
A comparison of the patient characteristics between the two trials is provided in Table 2. Both trials enrolled patients with vasopressor-dependent shock, who were also ventilator dependent. However, a higher number of patients received renal replacement therapy and had pneumonia in the APROCCHSS trial than in the ADRENAL trial. Whether more patients with acute respiratory distress syndrome were enrolled in the APROCCHSS trial and whether this was related to the survival benefit in the trial’s results has not been mentioned clearly. Both trials did not taper the dose of hydrocortisone during the 7-day treatment. The reported incidence of adverse events was considerably lower in the ADRENAL trial than in the APROCCHSS trial. The APROCCHSS trial reported a higher incidence of hyperglycemia in the hydrocortisone plus fludrocortisone group than in the placebo group (89.1% vs. 83.1%, P=0.002). Whether these results can be generalized to Asian populations remains a concern. Thus, additional large-scale clinical trials investigating the effects of hydrocortisone on Asian patients with septic shock are warranted.
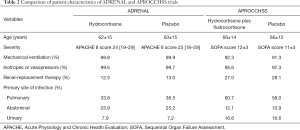
Full table
Conclusions
Low doses of hydrocortisone can shorten the episodes of septic shock and may decrease the severity of organ failure and increase survival rate. Identifying suitable patients and the optimal time to use hydrocortisone is the responsibility of the intensive care team presented during resuscitation of septic shock. For Asian populations, intensivists must carefully assess responses to hydrocortisone and monitor any possible adverse events. Additional large-scale clinical trials should examine Asian patients with septic shock to determine differences from the results of ADRENAL and APROCCHSS trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Reinhart K, Daniels R, Kissoon N, et al. Recognizing Sepsis as a Global Health Priority - A WHO Resolution. N Engl J Med 2017;377:414-7. [Crossref] [PubMed]
- Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med 2016;193:259-72. [Crossref] [PubMed]
- Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:775-87. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids for treating sepsis. Cochrane Database Syst Rev 2015.CD002243. [PubMed]
- Volbeda M, Wetterslev J, Gluud C, et al. Glucocorticosteroids for sepsis: systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 2015;41:1220-34. [Crossref] [PubMed]
- Venkatesh B, Finfer S, Cohen J, et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N Engl J Med 2018;378:797-808. [Crossref] [PubMed]
- Annane D, Renault A, Brun-Buisson C, et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N Engl J Med 2018;378:809-18. [Crossref] [PubMed]
- Fadel F, Andre-Gregoire G, Gravez B, et al. Aldosterone and Vascular Mineralocorticoid Receptors in Murine Endotoxic and Human Septic Shock. Crit Care Med 2017;45:e954-62. [Crossref] [PubMed]
- Investigators CS, Annane D, Cariou A, et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA 2010;303:341-8. [Crossref] [PubMed]
Cite this article as: Yeh YC, Chiu CT. ADRENAL trial versus APROCCHSS trial: to steroid or not to steroid? J Emerg Crit Care Med 2018;2:44.


