Platelet dysfunction in the perioperative and critical care setting
Background
Approximately 1×1011 platelets are produced by the cytoplasmic fragmentation of megakaryocytes each day (1). It has been argued that platelets—in many ways—behave more like cells than fragments. Studies suggest that thrombopoiesis can occur in the circulation, and platelets are capable of turning buds and extrusions into pro- and then pre-platelets, a process not normally expected for a simple cell fragment (2). Platelets can produce phospholipid microvesicles (100–1,000 nm), also called platelet microparticles (PMPs) or extracellular vesicles, with the stimulation of thrombin, collagen, or high shear stress with turbulent blood flow and during the apoptosis process. Unlike microparticles secreted by other cells, PMPs are not released through exocytosis, and have the distinct property of expressing the same antigens as the (parent) platelets (2). In addition, platelets can sense injured vascular sub-endothelium structures or infectious pathogens and respond to soluble molecules—including thrombin or thrombin-derived peptides—or bind to pathogens, either directly or indirectly, as immune complexes with antibodies or complement factors (2,3). Platelets are considered as one of the most short-lived ‘cells’ in human by a process called senescent platelet death (or apoptosis by others) that is highly regulated by intra-platelet signalling mechanisms (2,4) through thrombin activation (5), resulting in release of PMPs (6). Functionally, platelets play a pivotal role to control bleeding by adhering to an injured vessel wall and activating the coagulation factors.
Achieving haemostasis and, simultaneously, preventing arterial and venous thromboembolism (VTE) in the perioperative and critical care setting is challenging. With advances in our ability to measure platelet activity and function beyond platelet count alone, we are making substantial progress in our understanding of when and how platelets interact and cross-talk with the coagulation factors to achieve haemostasis and, conversely, contribute in the development of thrombosis after major surgery and trauma. In addition, platelets are increasingly recognized to play a role, as well as being affected by a variety of pathophysiological processes including inflammation and host defence (7). In this narrative review, we aimed to summarize the significance of platelet dysfunction and the possible roles of measuring platelet function in the perioperative and critical care setting.
Current state of managing platelet transfusion & function
To a large extent, bleeding and thrombosis are the different sides of the same coin. Current transfusion and thromboprophylaxis guidelines, including the National Blood Authority critical bleeding management tool, recommended platelet transfusion and withholding anticoagulants using a certain platelet count threshold in an attempt to reduce bleeding and unnecessary platelet transfusion (8-10). These guidelines stated that these recommendations were based on weak evidence with an emphasis that more research is needed. The obvious evidence gap in these guidelines is whether the platelet function—beyond the count alone—has any important clinical relevance and, therefore, should be considered in the medical decision-making in perioperative medicine.
Our understanding of platelet physiology has dramatically improved in the last few decades, primarily driven by the need to support the advances in percutaneous cardiac interventions. It is clear now that platelet activity or function cannot be considered as an “all or none” phenomenon. Three distinct major platelet activation pathways are well established (7,11), and each of these can be specifically inhibited by an antiplatelet agent, so much so that it is not rare to have patients treated with dual antiplatelet therapy (DAPT) and, in some acute cardiac care situations, even triple antiplatelet therapy. There is strong evidence to support such approach in reducing in-stent thrombosis and subsequent acute coronary events, especially when reperfusion is not satisfactory (12). More interestingly, large randomised controlled trials (RCTs) showed that aggressive DAPT or triple therapy can also have an ‘antiplatelet intensity-related’ protective effect on VTE (12), confirming the pivotal role of platelets in the pathogenesis of both venous and arterial thrombosis. Nevertheless, there is also a problem of variability in the pharmacokinetics and pharmacodynamics of anti-platelet therapy between individuals (e.g., aspirin resistance and clopidogrel CYP-2C19 enzyme pharmacogenomic poor-metaboliser in 30% and 40% of the population, respectively) (13). This has led to widespread use of platelet function testing in an attempt to individualise antiplatelet therapy, by adjusting the dose or type of antiplatelet drug between different patients as well as within the same individuals over the time, in cardiology practice.
Counterintuitive results of the antiplatelet therapy and platelet transfusion trials in the perioperative and critical care setting
Traditionally, most clinicians would rely on how long a patient has stopped taking antiplatelet therapy as a way to determine the degree of residual antiplatelet effect that may still exist, and whether the patient would be safe to undergo invasive procedures. Two recent landmark studies have challenged the validity of this simple intuition. The ATACAS RCT involving 2,100 patients tested the hypothesis that a single-dose of preoperative aspirin before coronary artery surgery would reduce a composite endpoint of death and thrombotic complications (non-fatal myocardial infarction, stroke, pulmonary embolism, renal failure, or bowel infarction) within 30 days after surgery. This trial did not find any significant differences in all clinical outcomes between the placebo and aspirin groups (14). The second study assessed the hypothesis that platelet transfusion for patients who were taking aspirin before haemorrhagic stroke could limit the extension of cerebral haemorrhage with better neurological outcomes. Surprisingly, the odds of death or dependence at 3 months were actually higher in the platelet transfusion group than in the standard care group (adjusted common odds ratio 2.1, 95% CI: 1.2–3.6; P=0.01) (15). In addition, 40 (42%) participants who received platelet transfusion had a serious adverse event during their hospital stay, compared to only 28 (29%) who received standard care. The ‘empirical’ nature of initiating an antiplatelet agent or platelet transfusion—without considering whether significant antiplatelet therapeutic effect already existed—could at least in part explain the rather counterintuitive results of these two landmark trials. Theoretically, we would like to know that both groups were well-balanced in their degree of platelet inhibition (by a platelet function analyser) at baseline; and in retrospect, platelet transfusion should only be reserved for those with a certain degree of antiplatelet activity on board at the time of stroke, instead of assuming that any patients who had been taking aspirin within 7 days of stroke would benefit from platelet transfusion without harms.
Viscoelastic blood tests in perioperative and critical care setting
Whole-blood viscoelastic blood tests are increasingly used to assess bleeding risk and guide transfusion in the perioperative and critical care setting (16-19). The initial clotting time (CT) on ROTEM® (or r-time on the thromboelastography®) is useful to detect the effect of unfractionated heparin (UFH) or low-molecular-weight-heparin (LMWH) or coagulation factor deficiency, while the maximum clot firmness (MCF) or maximum amplitude (MA) is more useful to delineate platelet activity or fibrinogen effect on the ROTEM® or thromboelastography®, respectively. The evidence to support their role, in particular the MCF or MA (17), to guide transfusion in cardiac surgery as well as to assess thrombotic risk (20) is gathering strength, and has now been incorporated in some official perioperative and critical care transfusion guidelines (18,21).
It is, however, important to understand the limitations of viscoelastic tests. These may include: (I) it does not detect the effects of hypothermia on coagulation factors activities and platelet dysfunction (as the sample is usually measured at 37 °C); (II) it does not measure the effects of hypocalcaemia on clot strength and platelet function when citrated blood samples are used for analysis; (III) it does not measure platelet adhesion (e.g., von Willebrand disease) or platelet aggregation problems (including the thrombotic risk of microangiopathic thrombotic (22), and—most important of all—it does not detect the effects of aspirin and clopidogrel in cardiac surgical patients (unless a TEG® Platelet Mapping module is used), because the activator used in viscoelastic tests would generate enough thrombin to activate phospholipase C (the dominant platelet activation pathway) overriding the inhibition of minor platelet activation pathways through the P2Y12 receptors and cyclooxygenase-1 (23). As such, whole-blood viscoelastic blood tests cannot be considered as the ‘panacea’ in the management of haemostasis and thrombosis and should also be used in conjunction with full blood count, coagulation blood tests and possibly also platelet function tests (23).
Platelet function tests in perioperative and critical care setting
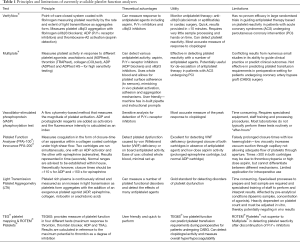
Excessive platelet activation (which may result in increased thromboembolism) can be implied by a number of platelet bioassays. These platelet-related assays and biomarkers are primarily used for research purposes currently and hence, we will not discuss their utility further in this review. Interested readers can refer to some of other reviews on this topic (24-26). Conversely, platelet function analysers that can detect suboptimal platelet function or effect of antiplatelet agents are much more widely used in a clinical environment. These platelet function analysers can be broadly divided into measuring the overall platelet function or the integrity of a specific platelet activation pathway. The most important limitation for almost all of these analysers is that the findings can be affected by thrombocytopenia and hence the results must be interpreted with the platelet count. The principles behind these analysers, their possible applications and limitations are summarised in Table 1 (23,27-29).
Full table
Platelet function tests in cardiac and non-cardiac surgery
Cardiac surgical patients are often treated with one or more antiplatelet agents before surgery, and bleeding requiring blood product transfusion is common. The use of platelet function test, in both preoperative and postoperative periods, to predict blood loss and transfusion requirements in cardiac surgery has been reported by a number of studies. While heterogeneity between multiple small studies (n<200 in most studies) exists, there is a reasonable signal to suggest that quantitative assessment of preoperative platelet inhibition (or dysfunction) has an association with perioperative blood loss and risk of requiring allogeneic blood transfusion [platelet function analyzer (PFA): area under the receiver-operating-characteristic curve (AUROC) 0.66; multiple electrode aggregation (MEA): AUROC 0.64–0.77] (30).
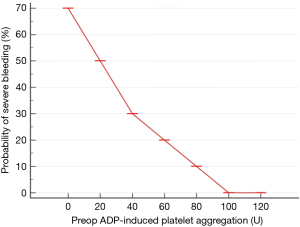
In addition, there is also a signal to suggest that there is a quantitative relationship between the risk of severe bleeding after cardiac surgery and the degree of platelet inhibition by ADP-blockers (31) (Figure 1). Although using platelet function assessment to assess risk of bleeding appears promising, there is substantial uncertainty about its utility, in particular whether only one, two or all three platelet activation pathways are equally important in determining perioperative blood loss and transfusion. Currently, platelet function test has been recommended (class II-b recommendation, grade-b evidence derived from a single randomized clinical trial or large non-randomized studies) by the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Cardiothoracic Anaesthesiology (EACTA) to guide timing of cardiac surgery if patients are treated with DAPT or P2Y12 blockers (32). Because most existing studies are relatively small, whether platelet function test can be used to predict blood loss and transfusion accurately, and hence can be used to guide platelet transfusion when bleeding occurs remains scientifically unproven. Although it makes clinical sense, the validity of using platelet function tests to guide timing of other invasive procedures, including epidural catheter insertion or neurosurgical procedures (33,34), in anaesthesia and perioperative medicine has also yet to be firmly supported by high-grade evidence.
Despite the promising hypothetical reasons to measure and optimize platelet function in both cardiology and cardiac surgical studies, the evidence to demonstrate the benefits of platelet function tests remains elusive (30,35). There are a few reasons why it is premature to abandon platelet function test in cardiac surgery completely. In the first instance, many studies have only assessed one specific type of platelet function analyser; it is possible that the other platelet function analysers may be better under different circumstances. Second, although still not completely proven, preliminary data suggests that platelet function test (supplemented by a viscoelastic test) may play a more important role to rationalize the choice of blood products during the period of active bleeding (36), when international normalised ratio (INR), activated partial thromboplastin time (aPTT), fibrinogen concentration and platelet count all relatively normal and the cause of ‘medical bleeding’ is uncertain. Third, platelet function test may also be considered in some special situations when any avoidable causes of bleeding or transfusion should be minimised, including those with difficulties in cross-match due to antibodies, and people who would not accept any form of allogeneic transfusion for a variety of reasons. Finally, it is most likely that platelet function test may be more accurate in predicting blood loss and transfusion in emergency cardiac surgical care or for those who are only treated with an antiplatelet agent stronger than aspirin alone (e.g., on clopidogrel or DAPT) and thus more indicated in emergency cardiac surgery when DAPT cannot be ceased prior to surgery (32). Indeed, the most promising studies on use of platelet function test to predict cardiac surgical bleeding appeared to involve primarily patients who were treated with ADP blockers or thienopyridines (37,38).
Improving platelet function during the perioperative period
While many drugs can inhibit platelet function through one of the three major platelet activation pathways (cyclooxygenase/thromboxane A2, P2Y12-ADP and thrombin), currently we do not have many interventions that can improve platelet function. Resveratrol, a polyphenol with strong antioxidant properties, is commonly found in plants and plant products such as berries, grapes and red wine (~14 mg/L). Its actions on sirtuins (silent information regulator—SIRT1, SIRT3, and SIRT4) gene expression is similar to caloric restriction, resulting in activation of telomerase to maintain telomere length (39). It is increasingly consumed as a nutritional supplement for cardiovascular, metabolic, and possibly anti-ageing benefits (39,40). In a large number of small-animal studies, intraperitoneal resveratrol administration has been shown to restore SIRT1 activity, attenuate hepatocyte injury, improve cardiac contractility, and ameliorate hypoxia-induced liver and kidney mitochondrial dysfunction following haemorrhagic injuries (41). A recent human in-vitro study showed that resveratrol can preserve function of stored platelets by improving mitochondrial function and reducing apoptosis (42).
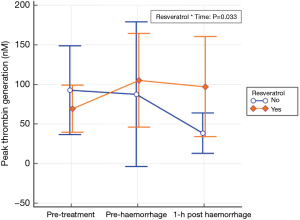
Using a double-blinded RCT design in a large animal (greyhound) model, our recent work showed that use of oral micronized resveratrol (10 mg/kg/d) for 1 week was associated with a substantial improvement in the animals’ tolerance to development of shock, requiring a much larger volume of haemorrhage to induce mean arterial blood pressure <40 mmHg compared to controls (67 vs. 51 mL/kg, Δ =16 mL/kg, 95% CI: 10–21) and there were significant improvement in platelet activity [measured by the maximal clot firmness (MCF) by the rotational thromboelastometry—ROTEM®] and thrombin generation (43) (Figure 2).
In addition, small phase-I human trials involved patients with colorectal cancer with hepatic metastases scheduled to undergo hepatectomy and colonic cancers preparing for surgery, oral resveratrol (at 5 and 1 g/d for 14 days before surgery, respectively) was well tolerated and associated with an increase in tumour apoptosis and a reduction in tumour proliferation (44,45). Therefore, there is a clinical need to confirm the potential benefits of resveratrol on coagulation by a phase II clinical trial before it can be tested as a perioperative therapy to prevent cancer recurrence in subsequent phase III cancer surgery trials.
Platelet dysfunction and thromboembolism in the critically ill
Cardiolipin release, platelet dysfunction and coagulopathy after traumatic brain injury (TBI)
TBI is a leading cause of death and disability in young people and accounts for approximately one-third of all trauma-related deaths. Coagulopathy is common in TBI and associated with poor clinical outcomes but the underlying pathogenesis remains poorly understood (46). Recent studies clearly showed that inhibition of platelet, in one or more of the three activation pathways, is common after severe TBI (47,48); and the degree of platelet dysfunction bears a quantitative relation with the severity of TBI in the absence of haemorrhagic shock or multisystem injury and antiplatelet therapy.
Cardiolipin is an anionic phospholipid primarily located in the leaflet of inner mitochondrial membrane of all cells and plays an important role in maintaining mitochondrial membrane fluidity. It regulates various mitochondrial processes including electron transport chain and programmed cell death (49). Cardiolipin maintains brain homeostasis, hence any alteration of its chemical structure or decrease in overall levels are implicated in the pathogenesis of various neurodegenerative diseases like Alzheimer’s and Parkinson’s’ disease. Recent experimental study using a murine TBI model showed that cardiolipin release from the brain could cause disruption of blood-brain-barrier and systemic and consumptive coagulopathy through activation of platelets (50-52). However, there are hardly any human clinical studies available that systematically investigated the effects of cardiolipin on coagulation, including its relationship to platelet dysfunction, after TBI. In addition, whether cardiolipin release leading to excessive platelet and thrombin activation is related to a reduction in plasma anti-thrombin levels (53,54)—which determines effectiveness of both UFH and LMWH—is also unknown. Understanding the mechanisms behind the development of TBI-induced coagulopathy is paramount to test and develop appropriate strategies to manage thrombocytopenia, in terms of platelet transfusion and thromboprophylaxis, after severe TBI. Further studies are therefore needed to determine whether the thrombocytopenic state after TBI is indeed prothrombotic, pro-bleeding, or both—changing with the time course of the disease.
Relationship between VTE and activation of platelets in patients with acquired coagulopathy
Acquired coagulopathy with thrombocytopenia, prolonged INR or aPTT is common in the perioperative setting. Yet, despite their underlying apparent bleeding propensity, the risk of thrombosis is high (55,56). In patients with chronic liver diseases, platelets appear to play a pivotal role in inducing both bleeding and thrombosis (57). In patients with coagulopathy in the perioperative setting (e.g., surgical emergencies and trauma) without chronic liver diseases, the contrition of excessive activation of platelets to development of bleeding requiring transfusion or thromboembolism is largely unknown. As such, the optimal haemostatic management including whether platelet transfusion is contraindicated for such patients remains highly controversial, and is one of the most important unanswered questions in VTE prophylaxis. Our recent work suggests that an increased clot strength on whole-blood viscoelastic test is associated with an increased risk of thromboembolic diseases in a variety of clinical situations (20), including patients with a prolonged INR or aPTT. We also found that excessive platelet activation in patients with acquired coagulopathy is associated with an increased risk of subsequent risk of clinical thromboembolic events (manuscript in preparation), consistent with the other evidence supporting the pivotal role of platelet in both arterial and venous thrombosis (12). In contrast to whole-blood viscoelastic tests, the currently available platelet function analysers are not useful to identify which patients at increased risk of thromboembolic events—with the exception in patients with heparin-induced thrombocytopenia syndrome (HITS) (58).
Conclusions and future research direction
Platelets are now known to process a variety of different physiological roles other than haemostasis alone, even though this latter function will remain as the main focus for most critical care physicians (2). Coagulation is an important area of perioperative and critical care medicine; and it is clear that the current practice of using platelet count and standard coagulation parameters alone to determine bleeding and thrombotic risk is inadequate, resulting in both false positives and negatives with important patient outcome and financial implications compared to in combination with viscoelastic and platelet function tests (15,16,59-61). Advances in quantitative assessment of platelet activity and/or function have allowed us to assess not only the effects of antiplatelet therapy, but also demonstrate the existence of platelet dysfunction without antiplatelet therapy in patients with severe TBI which has prognostic significance (46,52,62). Cardiolipin release from the brain appears to be responsible for the circulating microparticles that can explain why coagulopathy and thrombosis may coexist in patients with severe TBI; and excessive platelet activation in response to trauma and sepsis contribute to a high risk of thrombosis despite thromboprophylaxis in these patients (6).
Current evidence suggests that platelet function tests may assist clinicians in predicting their risk of bleeding especially in cardiac surgical patients treated preoperatively with thienopyridines, and platelet function may be improved using antioxidant polyphenol. Many of these hypotheses and emerging evidence do need further confirmation by adequately-powered phases II and III clinical trials. We are now entering the exciting stage of precision-medicine in the area of coagulation in perioperative and critical care (28). Ultimately, we would be able to use individualised-patient information—beyond abnormal platelet count, INR or aPTT alone—to avoid unnecessary platelet transfusion and omission of anticoagulant VTE prophylaxis in the critically ill.
Acknowledgements
Dr. Ho is funded by Raine Medical Research Foundation and WA Health through the Raine Clinical Research Fellowship. Both authors received a research grant from Haemonetics®. The content of this review and the decision to publish were not influenced by the funding agencies.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hartwig J, Italiano J Jr. The birth of the platelet. J Thromb Haemost 2003;1:1580-6. [Crossref] [PubMed]
- Garraud O, Cognasse F. Are Platelets Cells? And if Yes, are They Immune Cells? Front Immunol 2015;6:70. [Crossref] [PubMed]
- Menter DG, Kopetz S, Hawk E, et al. Platelet "first responders" in wound response, cancer, and metastasis. Cancer Metastasis Rev 2017;36:199-213. [Crossref] [PubMed]
- Gyulkhandanyan AV, Mutlu A, Freedman J, et al. Selective triggering of platelet apoptosis, platelet activation or both. Br J Haematol 2013;161:245-54. [Crossref] [PubMed]
- Leytin V, Allen DJ, Mykhaylov S, et al. Thrombin-triggered platelet apoptosis. J Thromb Haemost 2006;4:2656-63. [Crossref] [PubMed]
- Lopez E, Srivastava AK, Pati S, et al. Platelet-Derived Microvesicles: A Potential Therapy for Trauma-Induced Coagulopathy. Shock 2018;49:243-8. [Crossref] [PubMed]
- Gremmel T, Frelinger AL 3rd, Michelson AD. Platelet Physiology. Semin Thromb Hemost 2016;42:191-204. [Crossref] [PubMed]
- Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205-13. [Crossref] [PubMed]
- Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol 2013;30:270-382. [Crossref] [PubMed]
- Afshari A, Ageno W, Ahmed A, et al. ESA VTE Guidelines Task Force. European Guidelines on perioperative venous thromboembolism prophylaxis: Executive summary. Eur J Anaesthesiol 2018;35:77-83. [PubMed]
- Estevez B, Du X. New Concepts and Mechanisms of Platelet Activation Signaling. Physiology (Bethesda) 2017;32:162-77. [Crossref] [PubMed]
- Cavallari I, Morrow DA, Creager MA, et al. Frequency, Predictors, and Impact of Combined Antiplatelet Therapy on Venous Thromboembolism in Patients With Symptomatic Atherosclerosis. Circulation 2018;137:684-92. [Crossref] [PubMed]
- Wu AH. Drug metabolizing enzyme activities versus genetic variances for drug of clinical pharmacogenomic relevance. Clin Proteomics 2011;8:12. [Crossref] [PubMed]
- Myles PS, Smith JA, Forbes A, et al. Stopping vs. Continuing Aspirin before Coronary Artery Surgery. N Engl J Med 2016;374:728-37. [Crossref] [PubMed]
- Baharoglu MI, Cordonnier C, Al-Shahi Salman R, et al. PATCH Investigators. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet 2016;387:2605-13. [Crossref] [PubMed]
- Whiting P, Al M, Westwood M, et al. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost-effectiveness analysis. Health Technol Assess 2015;19:1-228. v-vi. [Crossref] [PubMed]
- Harahsheh Y, Ho KM. Viscoelastic point-of-care testing to guide transfusion and antithrombotic therapy in perioperative and critically ill patients: are all parameters created equal? Anaesth Intensive Care 2016;44:11-3. [PubMed]
- Roullet S, de Maistre E, Ickx B, et al. GIHP. Position of the French Working Group on Perioperative Haemostasis (GIHP) on viscoelastic tests: What role for which indication in bleeding situations? Anaesth Crit Care Pain Med 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Franchini M, Mengoli C, Cruciani M, et al. The use of viscoelastic haemostatic assays in non-cardiac surgical settings: a systematic review and meta-analysis. Blood Transfus 2018;16:235-43. [PubMed]
- Harahsheh Y, Ho KM. Use of viscoelastic tests to predict clinical thromboembolic events: A systematic review and meta-analysis. Eur J Haematol 2018;100:113-23. [Crossref] [PubMed]
- Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care 2016;20:100.
- Harahsheh Y, Ho KM. Thromboelastometry and thromboelastography failed to detect hypercoagulability in thrombotic microangiopathy. Anaesth Intensive Care 2016;44:520-1. [PubMed]
- Ho KM, Pavey W. Applying the cell-based coagulation model in the management of critical bleeding. Anaesth Intensive Care 2017;45:166-76. [PubMed]
- Yun SH, Sim EH, Goh RY, et al. Platelet Activation: The Mechanisms and Potential Biomarkers. Biomed Res Int 2016;2016:9060143.
- Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood 2017;130:1499-506. [Crossref] [PubMed]
- Laroche M, Dunois C, Vissac AM, et al. Update on functional and genetic laboratory assays for the detection of platelet microvesicles. Platelets 2017;28:235-41. [Crossref] [PubMed]
- Podda G, Scavone M, Femia EA, et al. Aggregometry in the settings of thrombocytopenia, thrombocytosis and antiplatelet therapy. Platelets 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Landry S, Tanguay JF, Lordkipanidzé M. Personalizing antiplatelet therapies: What have we learned from recent trials? Platelets 2018;29:131-9. [Crossref] [PubMed]
- Lordkipanidzé M. Platelet Function Tests. Semin Thromb Hemost 2016;42:258-67. [Crossref] [PubMed]
- Larsen JB, Hvas AM. Predictive Value of Whole Blood and Plasma Coagulation Tests for Intra- and Postoperative Bleeding Risk: A Systematic Review. Semin Thromb Hemost 2017;43:772-805. [Crossref] [PubMed]
- Malm CJ, Hansson EC, Åkesson J, et al. Preoperative platelet function predicts perioperative bleeding complications in ticagrelor-treated cardiac surgery patients: a prospective observational study. Br J Anaesth 2016;117:309-15. [Crossref] [PubMed]
- Task Force on Patient Blood Management for Adult Cardiac Surgery of the European Association for CardioThoracic Surgery (EACTS) and the European Association of Cardiothoracic Anaesthesiology (EACTA), Boer C, Meesters MI, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth 2018;32:88-120. [Crossref] [PubMed]
- Tanaka KA, Dietrich W. Is it time to implement preoperative platelet function testing before invasive procedures? Br J Anaesth 2011;107:842-3. [Crossref] [PubMed]
- Taylor LI, Dickerson JC, Dambrino RJ, et al. Platelet testing in flow diversion: a review of the evidence. Neurosurg Focus 2017;42:E5. [Crossref] [PubMed]
- Petricevic M, Vopjar T, Biocina B, et al. The predictive value of platelet function point-of-care tests for postoperative blood loss and transfusion in routine cardiac surgery: a systematic review. Thorac Cardiovasc Surg 2015;63:2-20. [PubMed]
- Weber CF, Görlinger K, Meininger D, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology 2012;117:531-47. [Crossref] [PubMed]
- Ranucci M, Colella D, Baryshnikova E, et al. Surgical and Clinical Outcome Research (SCORE) Group. Effect of preoperative P2Y12 and thrombin platelet receptor inhibition on bleeding after cardiac surgery. Br J Anaesth 2014;113:970-6. [Crossref] [PubMed]
- Schimmer C, Hamouda K, Sommer SP, et al. The predictive value of multiple electrode platelet aggregometry (multiplate) in adult cardiac surgery. Thorac Cardiovasc Surg 2013;61:733-43. [Crossref] [PubMed]
- Yamashita S, Ogawa K, Ikei T, et al. SIRT1 prevents replicative senescence of normal human umbilical cord fibroblast through potentiating the transcription of human telomerase reverse transcriptase gene. Biochem Biophys Res Commun 2012;417:630-4. [Crossref] [PubMed]
- Novelle MG, Wahl D, Diéguez C, et al. Resveratrol supplementation: Where are we now and where should we go? Ageing Res Rev 2015;21:1-15. [Crossref] [PubMed]
- Liu FC, Tsai YF, Tsai HI, et al. Anti-Inflammatory and Organ-Protective Effects of Resveratrol in Trauma-Hemorrhagic Injury. Mediators Inflamm 2015;2015:643763.
- Lannan KL, Refaai MA, Ture SK, et al. Resveratrol preserves the function of human platelets stored for transfusion. Br J Haematol 2016;172:794-806. [Crossref] [PubMed]
- Ho KM, Davis J, Raisis AL, et al. Effects of oral resveratrol supplementation on development of hemorrhagic shock and acute kidney injury: a randomized double-blinded controlled canine study. Shock 2017;47:74.
- Howells LM, Berry DP, Elliott PJ, et al. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila) 2011;4:1419-25. [Crossref] [PubMed]
- Patel KR, Brown VA, Jones DJ, et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res 2010;70:7392-9. [Crossref] [PubMed]
- Sun Y, Wang J, Wu X, et al. Validating the incidence of coagulopathy and disseminated intravascular coagulation in patients with traumatic brain injury–analysis of 242 cases. Br J Neurosurg 2011;25:363-8. [Crossref] [PubMed]
- Kutcher ME, Redick BJ, McCreery RC, et al. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg 2012;73:13-9. [Crossref] [PubMed]
- Castellino FJ, Chapman MP, Donahue DL, et al. Traumatic brain injury causes platelet adenosine diphosphate and arachidonic acid receptor inhibition independent of hemorrhagic shock in humans and rats. J Trauma Acute Care Surg 2014;76:1169-76. [Crossref] [PubMed]
- Paradies G, Petrosillo G, Paradies V, et al. Mitochondrial dysfunction in brain aging: role of oxidative stress and cardiolipin. Neurochem Int 2011;58:447-57. [Crossref] [PubMed]
- Tian Y, Salsbery B, Wang M, et al. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood 2015;125:2151-9. [Crossref] [PubMed]
- Zhao Z, Wang M, Tian Y, et al. Cardiolipin-mediated procoagulant activity of mitochondria contributes to traumatic brain injury-associated coagulopathy in mice. Blood 2016;127:2763-72. [Crossref] [PubMed]
- Zhang J, Jiang R, Liu L, et al. Traumatic brain injury-associated coagulopathy. J Neurotrauma 2012;29:2597-605. [Crossref] [PubMed]
- Connelly CR, Van PY, Hart KD, et al. Thrombelastography-Based Dosing of Enoxaparin for Thromboprophylaxis in Trauma and Surgical Patients: A Randomized Clinical Trial. JAMA Surg 2016;151:e162069. [Crossref] [PubMed]
- Louis SG, Van PY, Riha GM, et al. Thromboelastogram-guided enoxaparin dosing does not confer protection from deep venous thrombosis: a randomized controlled pilot trial. J Trauma Acute Care Surg 2014;76:937-42. [Crossref] [PubMed]
- Ho KM, Tan JA. Can the presence of significant coagulopathy be useful to exclude symptomatic acute pulmonary embolism? Anaesth Intensive Care 2013;41:322-7. [PubMed]
- Senzolo M, Sartori MT, Lisman T. Should we give thromboprophylaxis to patients with liver cirrhosis and coagulopathy? HPB (Oxford) 2009;11:459-64. [Crossref] [PubMed]
- Tapper EB, Robson SC, Malik R. Coagulopathy in cirrhosis - the role of the platelet in hemostasis. J Hepatol 2013;59:889-90. [Crossref] [PubMed]
- Slavik L, Svobodova G, Ulehlova J, et al. The advantages and limitations of impedance aggregometry in detection of heparin-induced thrombocytopenia. Clin Lab 2014;60:1319-24. [Crossref] [PubMed]
- Nogami K. The utility of thromboelastography in inherited and acquired bleeding disorders. Br J Haematol 2016;174:503-14. [Crossref] [PubMed]
- Della Corte A, Bancone C, Spadafora A, et al. Postoperative bleeding in coronary artery bypass patients on double antiplatelet therapy: predictive value of preoperative aggregometry. Eur J Cardiothorac Surg 2017;52:901-8. [Crossref] [PubMed]
- Bolliger D, Tanaka KA. Point-of-Care Coagulation Testing in Cardiac Surgery. Semin Thromb Hemost 2017;43:386-96. [Crossref] [PubMed]
- Herbert JP, Guillotte AR, Hammer RD, et al. Coagulopathy in the Setting of Mild Traumatic Brain Injury: Truths and Consequences. Brain Sci 2017;7. [Crossref] [PubMed]
Cite this article as: Ho KM, Harahsheh Y. Platelet dysfunction in the perioperative and critical care setting. J Emerg Crit Care Med 2018;2:48.