
Clinical practice guidelines for the management of central venous catheter for critically ill patients
Introduction
Central venous catheter (CVC) is one of the most commonly used interventions in the critically ill patients. Reasons for inserting a CVC include rapid administration of fluids during resuscitation periods, monitoring of hemodynamic status, administration of vasoconstrictors or veno-sclerotic drugs and, using large bore catheters, for the purposes of hemofiltration. Also, some drugs or fluids such as parental nutrition, potassium solution, strong vasoconstrictors and chemotherapy drugs must be given via CVC. However, CVC is an invasive technique and should be managed properly to minimize potential risks. Some catastrophic complications of CVC placement include pneumothorax, artery injury, blood stream infection, thrombosis, and human errors such as air embolism and unintentional guidewire embolization. Clinicians should weigh the risks and benefits before deciding to insert CVCs. However, such a widely used treatment tool lacks formal guidelines and the clinical practice patterns are heterogeneous. The Asian society of emergency and critical care medicine convened a consensus meeting and drafted a clinical practice guideline for the management of CVC. The guideline was developed under the framework of The Appraisal of Guidelines for Research & Evaluation II instrument (AGREE II).
Scope and purpose
The guideline aims to provide evidence based state-of-the-art guidelines for the management of CVC in the intensive care unit in all critically ill patients treated in the ICU. The guideline covers topics of indications and contraindications of CVC insertion, strategies to lower complications related to the CVC insertion, maintenance of CVC and prevention of CVC-related complications (e.g., thrombosis, blood stream infection). The purpose is to increase the benefits of CVC, while keeping risks at the lowest level. The views and preferences of the patients in ICUs were sought by literature review. If an intervention or treatment was unacceptable for patients or their family members, the recommendation of the intervention or treatment would be downgraded.
Stakeholder involvement
The guideline development group included intensivist, critical care nurses, personnel from infection control department and emergency physicians. The target users of the guideline included intensivist, critical care nurses, emergency physicians and policymakers. The guideline aimed to inform clinical decision making such as when to insert a CVC, should ultrasound be a routine for guiding CVC placement and what solution can be used to keep catheter patency. Also the guideline can be used for policy making such as nursing bundle for the prevention of catheter-related blood stream infection (CRBSI).
Development of recommendations
Electronic databases of CENTRAL, CINAHL, EMBASE, four Chinese databases (CBM, WANFANG DATA, CAJD, VIP Database) and Google Scholar were searched from inception to August 2017. The core search terms included “central venous catheter” and “critical care”. All relevant items were screened and reviewed.
The inclusion criteria were (I) clinical studies conducted in ICU; (II) the study investigated clinical questions related to the CVC; (III) systematic review and meta-analysis had the priority to be included. Studies were excluded if (I) they were duplicated report of the same work; (II) a meta-analysis that had been updated by a new one with more recent publications; (III) articles rather than original articles such as letters, reviews and commentaries. Review articles were reviewed manually to identify additional original studies. If there were no updated systematic review and meta-analysis, we would perform it by adding new studies.
The strengths and limitations of the body of evidence underlying a recommendation were clearly described. The risk of bias for included clinical studies were assessed from the aspects of study design, methodology limitations (sampling, blinding, allocation concealment, analytical methods), appropriateness/relevance of primary and secondary outcomes considered, consistency of results across studies, direction of results across studies, magnitude of benefit versus magnitude of harm, applicability to practice context (1,2).
The reviewers assessed each included study for the risk of bias under the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework (1,2). State-of-the-art instruments of quality assessment were used: Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 for studies of diagnostic accuracy (3,4), Cochrane for randomized controlled trials (RCTs) (5), and GRADE for observational studies that inform both therapy and prognosis questions. The GRADE evidence profile tables were added to each important clinical outcome. These important outcomes were risk of blood stream infection, lumen patency, thrombosis, artery injury and pneumothorax. The follow-up time was the period of the in-hospital stay. Based on the study methodologies and the 5 core GRADE domains of risk of bias, inconsistency, indirectness, imprecision, and other considerations (including publication bias), the quality of the evidence (or confidence in the estimate of the effect) was categorized as high, moderate, low, or very low. Recommendations were then formulated by using the modified Dephi method. These recommendations were designated as either strong or weak, taking into account an overall assessment of the evidence and a statement from the task force about the values and preferences that underlie the recommendations. We use the word “recommend” to indicate a strong recommendation and “suggest” to indicate a weak recommendation (Table 1).

Full table
The guideline will be updated in a 5-year interval by incorporating new evidence.
Results
We commend the use of catheter impregnation to prevent CRBSI (1A)
There is a Cochrane review updated in the year 2016 and this review provided state-of-the-art evidence for making recommendations (6). In the systematic review, a total of 57 studies were included into analysis, the summary results are shown in Table 2. Many study end points including CRBSI, catheter colonization, clinically diagnosed sepsis and all cause-mortality were evaluated. Most studies (42/57) reported CRBSI as the primary end-point. The quality of included RCTs was considered to be high because there was no impairment of the five domains. The result showed that catheter impregnation significantly reduced CRBSI as compared with non-impregnated catheters with a relative risk of 0.62 (95% CI: 0.52–0.74). The number needed to treat to benefit (NNTB) is 50. Because CRBSI is an important indicator of the quality of nosocomial infection control, we considered it as an important outcome. In contrast, the catheter colonization was considered as a less important outcome. The result showed that the impregnated catheters were able to reduce the risk of catheter colonization (RR 0.67; 95% CI: 0.59–0.76). A total of 12 studies reported the incidence of clinically diagnosed sepsis, but impregnated catheters were not able to reduce the risk (RR 1.00; 95% CI: 0.88–1.13). All-cause mortality was the most important outcome for the ICU patients, and a total of 10 studies reported this end-point. Although there was a marginal benefit of the impregnated catheter (RR 0.92; 95% CI: 0.80–1.07), statistical significance was not reached (Table 2).

Full table
However, the medical cost associated with the impregnated catheter was not reported in the Cochrane systematic review. Numerous studies have reported that the use of impregnated catheter could reduce CVC-related costs (7). The chlorhexidine-silver sulfadiazine (CHSS)-impregnated catheters were associated with lower CVC-related cost per day than standard catheters (€3.78 ± €4.45 vs. €7.28 ± €16.71, respectively) (8). The cost-effectiveness study of impregnated versus non-impregnated CVC were reported in an earlier systematic review. The economic performance of impregnated catheter was analyzed using a basic decision-analytic model and the results showed that there is an estimated cost-saving of 138.20 pounds for every patient who receives an impregnated CVC (9). Based on these evidence, we recommend the impregnated CVC for critically ill patients.
We suggest the use of real-time ultrasound guidance for subclavian or femoral vein insertion (2B), and recommend that for internal jugular vein (1A)
Common complications of CVC insertion included artery injury, pneumothorax, repeated attempts, hematoma and hemorrhage. Numerous efforts have been made in clinical investigations to minimize the risk associated with CVC insertion. The real-time ultrasound guidance was employed in many studies to improve the success rate and reduce complications.
A systematic review deposited in the Cochrane database included 13 studies investigating the use of US-guided subclavian or femoral vein insertion in adult population (10). The quality of evidence was low in 4 studies (11-14) involving subclavian and 1 studies (15) involving femoral vein, very low in three studies (16-18) involving subclavian vein (SV) for most outcomes, moderate for 1 study involving femoral vein and high for 1 study (19) involving SV. Overall, the quality of evidence was low and the US-guidance offers small gains in safety and quality when compared with an anatomical landmark technique for femoral vein (success on the first attempt) cannulation or subclavian (arterial puncture, haematoma formation) (Tables 3,4). For internal jugular vein (20), there was evidence that the use of two dimensional (2D) US-guidance reduced the number of participants with an inadvertent arterial puncture by 72% (4,388 participants in 22 studies, RR 0.28, 95% CI: 0.18 to 0.44; P value <0.00001, I2 =35%), and the risk of overall complications by 71% (2,406 participants in 14 studies, RR 0.29, 95% CI: 0.17 to 0.52; P<0.0001, I2 =57%). Overall success rates were modestly increased in overall groups at 12% (4,340 participants in 23 studies, RR 1.12, 95% CI: 1.08 to 1.17; P value <0.01, I2 =85%), and similar benefit was noted across all subgroups. Use of 2DUS increased the success rate at the first attempt by 57% (2,681 patients in 18 studies, RR 1.57, 95% CI: 1.36 to 1.82; P value <0.01, I2 =82%). The number of attempts was decreased in the overall population [3,302 participants in 16 studies, mean difference (MD) −1.19 attempts, 95% CI: −1.45 to −0.92; P value <0.00001, I2 =96%] and in all subgroups. The risk of haematoma formation was reduced by the use of 2DUS-guidance (overall reduction 73%, 3,233 participants in 13 studies, RR 0.27, 95% CI: 0.13 to 0.55; P value =0.0004, I2 =54%). The time to successful CVC insertion was decreased by 30.52 seconds in the 2DUS-guidance group (MD −30.52 seconds, 95% CI: −55.21 to −5.82; P value =0.02, I2 =97%).
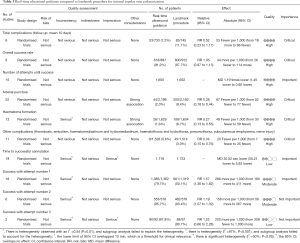
Full table
Full table
Although most of the randomised clinical trials carried out in this area have focused on the internal jugular vein and—to a lesser extent—on the SV it is clear that with growing clinical experience the benefits of ultrasound-guided venipuncture can be extended to all venous access sites, and this is especially true for SV (21). Yet, in a recent RCT a landmark control group was not included in the study because not using US in all patients was considered unethical (21). Moreover, in this study the US-guided infra-clavicular short-axis approach shows some clinical advantages, namely, a higher success rate, less complications and shorter insertion time than long-axis approach. The SV offers multiple advantages as a target for central venous access in the appropriately selected patient. The use of real-time US guidance for infraclavicular placement of SCV catheters allows for direct visualization of needle insertion and adjacent anatomical structures, as well as guidewire location and directionality, all of which can lead to decrease mechanical complications and improve cannulation success, compared to a landmark technique. In our opinion the current literature supports the use of the infraclavicular out-of-plane US-guided SCV catheterization as the preferred technique for cannulation of SV when compared to landmark approach and a solid alternative to cannulation of IJVs.
US usefulness in particular conditions such as obesity should also be considered. Obesity has been described as a risk factor for unsuccessful central venous cannulation or complications; thus, a technique more reliable than one based on anatomic landmarks only is recommended (22). The main findings of the present study are that (1) the anatomic variability of IJV was frequent in morbidly obese patients and (2) a diameter of IJV<10 mm was predictive of difficult positioning, whereas a diameter of IJV <6 mm was predictive of unsuccessful positioning, thus requiring an alternative access.
We suggest the use of real-time color Doppler ultrasound (CDUS) guidance on central venous catheterization for adult and pediatric patients (2C)
The usefulness of CDUS was investigated separately in the Brass’s systematic review (20). The chance of success at the first attempt was increased by 58% in the CDUS group (199 participants in 4 studies, RR 1.58, 95% CI: 1.02 to 2.43; P=0.04, I2 =57%). The total numbers of perioperative and postoperative complications/adverse events were similar (93 patients in 3 studies, RR 0.52, 95% CI: 0.16 to 1.71; P=0.28). The overall success rate (289 patients in 7 studies, RR 1.09, 95% CI: 0.95 to 1.25; P=0.20). Other outcomes such as the overall number of participants with an arterial puncture (213 participants in 6 studies, RR 0.61, 95% CI: 0.21 to 1.73; P=0.35), the total number of attempts until success (69 patients in 2 studies, MD −0.63, 95% CI: −1.92 to 0.66; P=0.34) and time to successful cannulation (five trials, 214 patients in 5 studies, each using a different definition for this outcome; MD 62.04 seconds, 95% CI: −13.47 to 137.55; P=0.11) were comparable in the Doppler ultrasound group versus landmark group (Table 5).
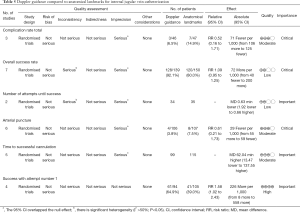
Full table
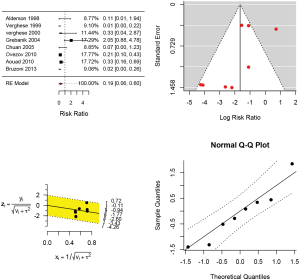
In pediatric patients (Table 6), a recent systematic review involving 8 RCTs were identified (23). The study involved 760 children and infants. The Jadad score was employed for the assessment of the risk of bias (24). One study (25) was scored 2 points, six studies (26-31) were scored 3 points and only one study (32) was scored 4. The forest plot (Figure 1) showed that real-time ultrasound guided CVC insertion was able to reduce the risk of CVC insertion failure (RR 0.19; 95% CI: 0.06–0.60). However, the quality of evidence was downgraded due to imprecision and potential publication bias (funnel plot) (33,34). US-guided CVC insertion could also help to decrease the mean number of attempts required (5 studies; difference in number −1.26; 95% CI: −1.711 to −0.812; P<0.001) and the risk of accidental arterial puncture (8 studies; RR 0.359; 95% CI: 0.118–1.093; P=0.071). However, US-guided CVC insertion was not associated with a significant difference in time required for CVC placement (4 studies; difference in minutes: −1.123, 95% CI: −2.600 to 0.353; P=0.136). In conclusion, the US-guided CVC insertion is able to increase the success rate, decrease the number of attempts and the arterial puncture, but will not increase the time for CVC placement. Although the evidence is low, we considered the accidental arterial puncture was an important outcome and there was no significant risk associated with the US, and we strongly recommend using US-guided CVC insertion.
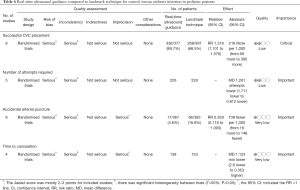
Full table
We suggest not to use heparin for the maintenance of CVC patency (2A)
The patency of CVC is of vital importance for its functionality. Thus, strenuous efforts have been made to keep the CVC patency in critically ill patients. We identified several systematic reviews of RCTs comparing heparin and saline in maintaining CVC patency (35,36). We choose the most updated one to make the recommendation (35). The primary purpose of the use of heparin or normal saline was to maintain CVC patency, thus the catheter occlusion was used as the primary end point in the majority of studies. However, catheter occlusion was not patient-important outcome, and the clinical importance was considered as moderate. There were 12 studies reporting this end point and the quality of the evidence was considered as high. Overall, heparin was not able to reduce the risk of catheter occlusion as compared with normal saline (RR 1.21; 95% CI: 0.91–1.61). Two studies reported the Maneuver needed and the results were comparable between the two groups (RR 1.24; 95% CI: 0.71–2.16). The quality of the studies was downgraded because of the confidence interval was wide. The incidence of heparin-induced thrombocytopenia was comparable between heparin and normal saline groups (RR 1.33; 95% CI: 0.09–18.54). The quality was low because there was potential publication bias and wide confidence interval. Three studies reported the incidence of hemorrhage and there was no statistical difference between the two groups. In conclusion, we do not suggest routine use of heparin for CVC patency (Table 7).

Full table
We suggest the use contrast-enhanced ultrasound (CEUS) for the confirmation of central venous catheter placement (2B)
There were no RCTs directly investigating the impact of CEUS on patient-important outcomes. Thus, the potential influence of CEUS was inferred from the diagnostic accuracy of the test, and potential impact of false positive (FP), true positive (TP), false negative (FN) and true negative (TN) on patient-important outcomes (37,38). For all these types of outcomes, we considered FP as critical importance because FP may prompt changing or repositioning of the catheter. Changing catheter carries all risks associated with CVC insertion such as pneumothorax, artery injury and hemorrhage. Thus, a good diagnostic tool should low the risk rate of FP.
Systematic search identified a systematic review and meta-analysis published in 2017 (39). The study included 5 original studies exploring the diagnostic accuracy of CEUS in confirming CVC placement (40-44). A total of 572 patients were included, and the pooled sensitivity and specificity were 72% (95% CI: 44–91%) and 100% (99–100%), respectively. The FP rate was 0.5% in the overall studies. However, there is no RCT directly investigating the impact of CEUS on patient-important outcomes such as mortality, hemorrhage, blood stream infection and thrombosis. Furthermore, the cost of CEUS was high and CEUS carries potential risks of contrast agent allergy. Thus, we make a weak recommendation for the use of CEUS to confirm CVC placement (Table 8).

Full table
We recommend the use of bedside ultrasound together with agitated or non-agitated normal saline to confirm CVC position (1C)
Since the CEUS is expensive and its impact on patient-important outcomes is unclear, the use of bedside ultrasound without contrast agents can also be used for the confirmation of catheter position in vascular and cardiac views. Normal saline with and without agitation can be used to, but is not mandatory, facilitate the identification of the catheter. There were numerous cohort studies being performed in this field (42,45-52), which has been summarized in a systematic review and meta-analysis (53). A total of 15 studies with 1,553 CVC insertions were identified, which resulted in a pooled sensitivity and specificity of catheter malposition by ultrasound of 0.82 (95% CI: 0.77–0.86) and 0.98 (95% CI: 0.97–0.99), respectively, corresponding to pooled positive and negative likelihood ratios of 31.12 (14.72–65.78) and 0.25 (0.13-0.47), respectively (Table 9). The diagnostic of ultrasound for pneumothorax detection was nearly 100% in the participating studies. The mean time required for bedside ultrasound confirmation of CVC was 5.6 minutes, which was significantly shorter than a time to chest radiograph completion of 63.9 minutes and a mean time to interpretation of 143.4 minutes. The quality of evidence was downgraded due to high risk of bias and clinical heterogeneity. Again, there was no RCT directly examining the impact of bedside ultrasound on patient-important outcomes such as mortality, pneumothorax requiring chest tube insertion and catheter malfunction (Table 10).

Full table
Full table
We suggest using subclavian site for CVC insertion (2C)
The three major sites for CVC insertion are internal jugular, femoral and subclavian sites. The choices of the insertion sites have been studied in many clinical trials. There were two RCTs and approximately 10 cohort studies being conducted in this field (54-56). A systematic review and meta-analysis was identified from the literature, investigating the impact of CVC insertion site on the risk of CRBSI (57). The results of systematic review are shown in Table 5. The femoral site showed similar risk of CRBSI as that of subclavian site, and the evidence was considered as of very low quality because of serious inconsistency and imprecision. While RCT did not report difference on the risk of CRBSI for femoral versus internal jugular veins, cohort studies showed higher risk of CRBSI in femoral versus internal jugular veins (RR 2.16; 95% CI: 1.44–3.22). With respect to the deep vein thrombosis (DVT), there was no significant difference in femoral versus subclavian/internal jugular veins. Two RCTs investigated mechanical complications of CVC influenced by insertion site (58,59). When internal jugular vein was compared with the SV, there was no difference in mechanical complications (RR 1.00; 95% CI: 0.21–4.84) (60). The evidence was downgraded by serious indirectness because one study enrolled patients requires chemotherapy (58). A recent mega-trial involving 3,027 patients showed that there were 8, 20, and 22 primary CRBSIs in the subclavian, jugular, and femoral groups, respectively (1.5, 3.6, and 4.6 per 1,000 catheter-days; P=0.02) (61). When the three arms were compared in pairwise fashion, the femoral group had significantly higher risk of CRBSI than that in the subclavian group (hazard ratio, 3.5; 95% CI, 1.5–7.8; P=0.003), and the jugular group had higher risk than that in the subclavian group (HR: 2.1; 95% CI, 1.0–4.3; P=0.04). However, the subclavian group showed higher rate of pneumothorax requiring chest tube insertion. A recent meta-analysis updated in 2017 showed that internal jugular (RR 2.25; 95% CI: 1.84–2.75; I2 =0%) and femoral (RR 2.92; 95% CI: 2.11–4.04); I2 =24%) had higher risk of colonization as compared with subclavian site (62). CRBSI was comparable for internal jugular and subclavian. Femoral site had higher risk of CRBSI than subclavian (RR 2.44; 95% CI: 1.25–4.75; I2 =61%), and internal jugular had lower risk of CRBSI than femoral (RR 0.55; 95% CI: 0.34–0.89; I2 =61%). Due to the benefit and risk of subclavian insertion site, we make a weak recommendation for it (Table 11).
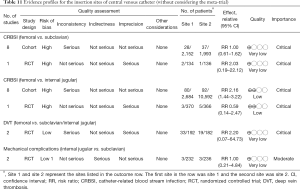
Full table
However, the choice of insertion site must be balanced with the experience of the operator and the clinical situation. User experience of the different sites of insertion will have a profound effect on the safest route in any given situation, balancing the complications of insertion with the complications over time related to the site of insertion. For example, anesthetists may become very proficient in the use of internal jugular catheterization because of the nature and access of this site during surgery and the inability of excluding possible pneumothorax following placement in the operating room. Additionally, ultrasound assisted insertion is easier taught for the internal jugular site and for many insertions this will be of necessity performed by junior doctors within their learning curve.
We suggest not using heparin-bonded catheters or warfarin to prevent CVC-related DVT in children (2D)
One important complication of CVC is the DVT. When the thrombus detached from the CVC insertion site, it can cause pulmonary embolism. The latter medical condition can be life-threatening. Pediatric patients are a specific group of population that need attention. Up to date, there are several RCTs investigating strategies to prevent DVT in children (63-72). These strategies included heparin-bounded CVC, unfractionated heparin, low molecular heparin, warfarin, antithrombin concentrate and nitroglycerin (73). However, none of these strategies were found to be able to reduce the risk of DVT (Table 6). Many of these included studies were not conducted in the ICU (65-68,72), compromising its directness to inform ICU staffs. Overall, the evidence underlying the DVT prevention was considered to be very low and we suggest not using these strategies or drugs to prevent DVT in children (Table 12).
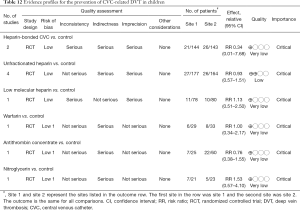
Full table
We recommend the implementation of central-line bundles to reduce the risk of CRBSI for adult, pediatric and neonatal ICUs (1B)
Since the presence of CVC has been identified as an important risk factor for CRBSI, implementation of central-line bundles is important to reduce the relative risk. There is no definitive consensus on specific procedures of central-line bundles and large variations exist across studies. A complete central-line bundle includes insertion bundle and maintenance bundle. The former included maximum barrier precaution (handwashing, wearing a cap, mask, sterile gown and gloves), skin-cleaning with chlorhexidine or povidone ionide, complete CVC cart that contain all necessary supplies for insertion a CVC, hand hygiene, Sterile dressing or gauze, use of CVC insertion checklist, Optimal CVC site (e.g., avoid femoral vein in adult). The latter includes Hand hygiene, Needle free connector, Infusion sets labeled, Replacement sets in predefined interval, label date of CVC insertion, Handling of CVC with sterile gauze-alcohol solution (74-81). In patients not receiving blood, blood products or fat emulsions, replace administration sets that are continuously used, including secondary sets and add-on devices, no more frequently than at 96-hour intervals, but at least every 7 days (82). Category IA (PMID: 21511081) Replace tubing used to administer blood, blood products, or fat emulsions (those combined with amino acids and glucose in a 3-in-1 admixture or infused separately) within 24 hours of initiating the infusion (82). Replace tubing used to administer propofol infusions every 6 or 12 hours, when the vial is changed, per the manufacturer
An updated systematic review and meta-analysis involving 79 original studies was included for making the recommendation (83). Most of the 79 studies were before-and-after study that the prevalence of CRBSI was compared before and after the implementation of CVC bundles. When data from all 79 studies were pooled with a random-effects model, the incidence risk ratio was 0.44 (95% CI: 0.39–0.50) favoring the bundle group. Similar results were obtained in adult ICU (IRR 0.45; 95% CI: 0.39–0.52), pediatric ICU (IRR 0.58; 95% CI: 0.48–0.71) and neonate ICU (IRR 0.47; 95% CI: 0.38–0.59). There were two clustered RCTs having been conducted. Speroff T and colleagues reported that the CLABSI rate was 2.42 per 1,000 catheter days at baseline and 2.73 at 18 months (P=0.59) (84). A clustered RCT enrolling 45 ICUs reported that while the baseline CRBSI rate was comparable between the intervention and control group (4.48 vs. 2.71 per 1,000 central line days; P=0.28), the infection rate declined to 1.33 in the intervention group compared to 2.16 in the control group (incidence rate ratio 0.19; P=0.003; 95% CI: 0.06–0.57) (85). Because there was significant heterogeneity among component studies (I2 =89% for all ICUs, 67%, 18% and 18% for adult, pediatric and neonate ICUs, respectively), and nearly all these studies were not RCT, the grade of evidence was downgraded to moderate (B). However, due to potential benefits and risks of bundle implementation, all experts believed the bundle implementation should be strongly recommended.
We suggest skin antisepsis with chlorhexidine throughout in-dwelling period for reducing CVC-related infections (2D)
It is proposed that the CVC-related infections are caused by insertion site contamination, and the following colonization on external surface of the catheter. Thus, it is rationale to deduce that skin antisepsis throughout the in-dwelling period can be effective in reducing CVC-related infections. Many RCTs have been conducted to investigate whether skin antisepsis was effective in reducing CVC-related infection (86-94). The three major antiseptic agents reported in the literature are chlorhexidine, iodine and alcohol. Antiseptic agents were applied both before catheter insertion and regularly thereafter during the in-dwelling period. The frequency of skin cleansing ranged from 24 to 72 h across these studies.
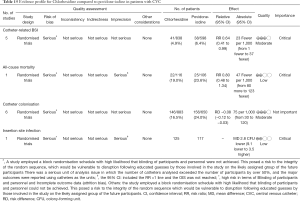
These studies were summarized in a systematic review and meta-analysis (95). A total of 13 studies were eligible for the analysis (Tables 13-16). The overall quality of included studies was considered to be low. Study endpoints such as catheter-related BSI, septicaemia, catheter colonisation and number of patients who required systemic antibiotics were not significantly different among all these agents. There was weak evidence (the level of evidence was downgraded due to imprecision and the risk of bias) that chlorhexidine may reduce the risk of CRBSI [RR of 0.64, 95% CI: 0.41–0.99; absolute risk reduction (ARR) 2.30%, 95% CI: 0.06–3.70%] and catheter colonization (RR of 0.68, 95% CI: 0.56–0.84; ARR 8%, 95% CI: 3–12%; 5 studies involving 1,533 catheters, downgraded for indirectness, risk of bias and inconsistency) as compared with povidone-iodine. Other head-to-head comparisons such as alcoholic chlorhexidine versus aqueous povidone-iodine, aqueous chlorhexidine versus aqueous povidone-iodine and alcoholic chlorhexidine versus alcoholic povidone-iodine showed no clear difference in CRBSI and mortality (95). In conclusion, the evidence is very low and skin antisepsis with chlorhexidine may provide protective effect against catheter colonization and CRBSI.

Full table

Full table
Full table

Full table
We recommend a differential time to positivity (DTP) of blood cultures from CVC and peripheral vein of 120 minutes to diagnose CRBSI (1B)
CRBSI is an important cause of morbidity and mortality and is potentially preventable. One challenge in the management of CRBSI is the correct diagnosis. In a critically ill patient with suspected infection, CRBSI should be suspected in the presence of a CVC. Blood samples should be sent for blood cultures. There is plenty of evidence showing that a DTP of blood cultures of 120 minutes has high sensitivity and specificity in diagnosing CRBSI. García and colleagues used DTP >120 min as a cutoff point, and correctly diagnosed 12 out of 15 CR-BSI cases (sensitivity 80%, specificity 99%, PPV 92%, NPV 98%) (96). Similar results were replicated in other studies (97-99). However, there is lack of evidence that such a high accuracy can be translated to benefits of patient important outcomes.
Editorial independence
There was no fund for the guideline development, and the members of the guideline development group declared no conflict of interest. The guideline was reviewed by independent reviewers.
Acknowledgements
We thank Giulia Adriano, Master degree in CRBSI control for the supervision.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ 2008;336:1049-51. [Crossref] [PubMed]
- Wade R, Corbett M, Eastwood A. Quality assessment of comparative diagnostic accuracy studies: our experience using a modified version of the QUADAS-2 tool. Res Synth Methods 2013;4:280-6. [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Lai NM, Chaiyakunapruk N, Lai NA, et al. Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults. Cochrane Database Syst Rev 2016;3:CD007878. [PubMed]
- Shorr AF, Humphreys CW, Helman DL. New choices for central venous catheters: potential financial implications. Chest 2003;124:275-84. [Crossref] [PubMed]
- Lorente L, Lecuona M, Jiménez A, et al. Chlorhexidine-silver sulfadiazine-impregnated venous catheters save costs. Am J Infect Control 2014;42:321-4. [Crossref] [PubMed]
- Hockenhull JC, Dwan K, Boland A, et al. The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation. Health Technol Assess 2008;12:iii-iv, xi-xii, 1-154.
- Brass P, Hellmich M, Kolodziej L, et al. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev 2015;1:CD011447. [PubMed]
- Palepu GB, Deven J, Subrahmanyam M, et al. Impact of ultrasonography on central venous catheter insertion in intensive care. Indian J Radiol Imaging 2009;19:191-8. [Crossref] [PubMed]
- Alic Y, Torgay A, Pirat A. Ultrasound-guided catheterization of the subclavian vein: a prospective comparison with the landmark technique in ICU patients. Crit Care 2009;13:198. [Crossref] [PubMed]
- Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med 2011;39:1607-12. [Crossref] [PubMed]
- Farrell J, Gellens M. Ultrasound-guided cannulation versus the landmark-guided technique for acute haemodialysis access. Nephrol Dial Transplant 1997;12:1234-7. [Crossref] [PubMed]
- Branger B, Zabadani B, Vecina F, et al. Continuous guidance for venous punctures using a new pulsed Doppler probe: efficiency, safety. Nephrologie 1994;15:137-40. [PubMed]
- Branger B, Dauzat M, Zabadani B, et al. Pulsed Doppler sonography for the guidance of vein puncture: a prospective study. Artif Organs 1995;19:933-8. [Crossref] [PubMed]
- Gualtieri E, Deppe SA, Sipperly ME, et al. Subclavian venous catheterization: greater success rate for less experienced operators using ultrasound guidance. Crit Care Med 1995;23:692-7. [Crossref] [PubMed]
- Mansfield PF, Hohn DC, Fornage BD, et al. Complications and failures of subclavian-vein catheterization. N Engl J Med 1994;331:1735-8. [Crossref] [PubMed]
- Bold RJ, Winchester DJ, Madary AR, et al. Prospective, randomized trial of Doppler-assisted subclavian vein catheterization. Arch Surg 1998;133:1089-93. [Crossref] [PubMed]
- Brass P, Hellmich M, Kolodziej L, et al. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev 2015;1:CD006962. [PubMed]
- Vezzani A, Manca T, Brusasco C, et al. A randomized clinical trial of ultrasound-guided infra-clavicular cannulation of the subclavian vein in cardiac surgical patients: short-axis versus long-axis approach. Intensive Care Med 2017;43:1594-601. [Crossref] [PubMed]
- Brusasco C, Corradi F, Zattoni PL, et al. Ultrasound-guided central venous cannulation in bariatric patients. Obes Surg 2009;19:1365-70. [Crossref] [PubMed]
- Lau CS, Chamberlain RS. Ultrasound-guided central venous catheter placement increases success rates in pediatric patients: a meta-analysis. Pediatr Res 2016;80:178-84. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Alderson PJ, Burrows FA, Stemp LI, et al. Use of ultrasound to evaluate internal jugular vein anatomy and to facilitate central venous cannulation in paediatric patients. Br J Anaesth 1993;70:145-8. [Crossref] [PubMed]
- Verghese ST, McGill WA, Patel RI, et al. Ultrasound-guided internal jugular venous cannulation in infants: a prospective comparison with the traditional palpation method. Anesthesiology 1999;91:71-7. [Crossref] [PubMed]
- Verghese ST, McGill WA, Patel RI, et al. Comparison of three techniques for internal jugular vein cannulation in infants. Paediatr Anaesth 2000;10:505-11. [Crossref] [PubMed]
- Chuan WX, Wei W, Yu L. A randomized-controlled study of ultrasound prelocation vs anatomical landmark-guided cannulation of the internal jugular vein in infants and children. Paediatr Anaesth 2005;15:733-8. [Crossref] [PubMed]
- Grebenik CR, Boyce A, Sinclair ME, et al. NICE guidelines for central venous catheterization in children. Is the evidence base sufficient? Br J Anaesth 2004;92:827-30. [Crossref] [PubMed]
- Aouad MT, Kanazi GE, Abdallah FW, et al. Femoral vein cannulation performed by residents: a comparison between ultrasound-guided and landmark technique in infants and children undergoing cardiac surgery. Anesth Analg 2010;111:724-8. [Crossref] [PubMed]
- Bruzoni M, Slater BJ, Wall J, et al. A prospective randomized trial of ultrasound- vs landmark-guided central venous access in the pediatric population. J Am Coll Surg 2013;216:939-43. [Crossref] [PubMed]
- Ovezov A, Zakirov I, Vishnyakova M. Effectiveness and safety of the internal jugular vein catheterization in pediatrics: ultrasound navigation vs anatomical landmarks (A prospective, randomized, double-blind study). Intensive Care Med 2010;36:S275.
- Anzures-Cabrera J, Higgins JP. Graphical displays for meta-analysis: An overview with suggestions for practice. Res Synth Methods 2010;1:66-80. [Crossref] [PubMed]
- Wang MC, Bushman BJ. Using the normal quantile plot to explore meta-analytic data sets. Psychological Methods 1998;3:46-54. [Crossref]
- Zhong L, Wang HL, Xu B, et al. Normal saline versus heparin for patency of central venous catheters in adult patients - a systematic review and meta-analysis. Crit Care 2017;21:5. [Crossref] [PubMed]
- Bradford NK, Edwards RM, Chan RJ. Heparin versus 0.9% sodium chloride intermittent flushing for the prevention of occlusion in long term central venous catheters in infants and children. Cochrane Database Syst Rev 2015.CD010996. [PubMed]
- Gopalakrishna G, Mustafa RA, Davenport C, et al. Applying Grading of Recommendations Assessment, Development and Evaluation (GRADE) to diagnostic tests was challenging but doable. J Clin Epidemiol 2014;67:760-8. [Crossref] [PubMed]
- Chen Y. Rationales, methods, challenges and development tendency of using GRADE in systematic reviews of diagnostic accuracy tests. Chinese Journal of Evidence-Based Medicine 2014;14:1402-6.
- Bou Chebl R, Kiblawi S, El Khuri C, et al. Use of Contrast-Enhanced Ultrasound for Confirmation of Central Venous Catheter Placement: Systematic Review and Meta-analysis. J Ultrasound Med 2017;36:2503-10. [Crossref] [PubMed]
- Vezzani A, Brusasco C, Palermo S, et al. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med 2010;38:533-8. [Crossref] [PubMed]
- Cortellaro F, Mellace L, Paglia S, et al. Contrast enhanced ultrasound vs chest x-ray to determine correct central venous catheter position. Am J Emerg Med 2014;32:78-81. [Crossref] [PubMed]
- Weekes AJ, Johnson DA, Keller SM, et al. Central vascular catheter placement evaluation using saline flush and bedside echocardiography. Acad Emerg Med 2014;21:65-72. [Crossref] [PubMed]
- Weekes AJ, Keller SM, Efune B, et al. Prospective comparison of ultrasound and CXR for confirmation of central vascular catheter placement. Emerg Med J 2016;33:176-80. [Crossref] [PubMed]
- Efune B, Keller SM, Ghali S, et al. Prospective observational study comparing ultrasound with chest X-ray for risk assessment for central vascular catheter tip misplacement and pneumothorax complications. Acad Emerg Med 2015;22:S213.
- Zanobetti M, Coppa A, Bulletti F, et al. Verification of correct central venous catheter placement in the emergency department: comparison between ultrasonography and chest radiography. Intern Emerg Med 2013;8:173-80. [Crossref] [PubMed]
- Matsushima K, Frankel HL. Bedside ultrasound can safely eliminate the need for chest radiographs after central venous catheter placement: CVC sono in the surgical ICU (SICU). J Surg Res 2010;163:155-61. [Crossref] [PubMed]
- Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med 2013;39:1932-7. [Crossref] [PubMed]
- Baviskar AS, Khatib KI, Bhoi S, et al. Confirmation of endovenous placement of central catheter using the ultrasonographic "bubble test". Indian J Crit Care Med 2015;19:38-41. [Crossref] [PubMed]
- Meggiolaro M, Scatto A, Zorzi A, et al. Confirmation of correct central venous catheter position in the preoperative setting by echocardiographic "bubble-test". Minerva Anestesiol 2015;81:989-1000. [PubMed]
- Duran-Gehring PE, Guirgis FW, McKee KC, et al. The bubble study: ultrasound confirmation of central venous catheter placement. Am J Emerg Med 2015;33:315-9. [Crossref] [PubMed]
- Gekle R, Dubensky L, Haddad S, et al. Saline Flush Test: Can Bedside Sonography Replace Conventional Radiography for Confirmation of Above-the-Diaphragm Central Venous Catheter Placement? J Ultrasound Med 2015;34:1295-9. [Crossref] [PubMed]
- Wen M, Stock K, Heemann U, et al. Agitated saline bubble-enhanced transthoracic echocardiography: a novel method to visualize the position of central venous catheter. Crit Care Med 2014;42:e231-3. [Crossref] [PubMed]
- Ablordeppey EA, Drewry AM, Beyer AB, et al. Diagnostic Accuracy of Central Venous Catheter Confirmation by Bedside Ultrasound Versus Chest Radiography in Critically Ill Patients: A Systematic Review and Meta-Analysis. Crit Care Med 2017;45:715-24. [Crossref] [PubMed]
- Theaker C, Juste R, Lucas N, et al. Comparison of bacterial colonization rates of antiseptic impregnated and pure polymer central venous catheters in the critically ill. J Hosp Infect 2002;52:310-2. [Crossref] [PubMed]
- Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA 2001;286:700-7. [Crossref] [PubMed]
- Parienti JJ, Thirion M, Mégarbane B, et al. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: a randomized controlled trial. JAMA 2008;299:2413-22. [Crossref] [PubMed]
- Marik PE, Flemmer M, Harrison W. The risk of catheter-related bloodstream infection with femoral venous catheters as compared to subclavian and internal jugular venous catheters: a systematic review of the literature and meta-analysis. Crit Care Med 2012;40:2479-85. [Crossref] [PubMed]
- Biffi R, Orsi F, Pozzi S, et al. Best choice of central venous insertion site for the prevention of catheter-related complications in adult patients who need cancer therapy: a randomized trial. Ann Oncol 2009;20:935-40. [Crossref] [PubMed]
- Wang SX. Complications of two different central venous insertion sites. Chinese Journal of Practical Nursing 2006;22:46-7.
- Ge X, Cavallazzi R, Li C, et al. Central venous access sites for the prevention of venous thrombosis, stenosis and infection. Cochrane Database Syst Rev 2012.CD004084. [PubMed]
- Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular Complications of Central Venous Catheterization by Insertion Site. N Engl J Med 2015;373:1220-9. [Crossref] [PubMed]
- Arvaniti K, Lathyris D, Blot S, et al. Cumulative Evidence of Randomized Controlled and Observational Studies on Catheter-Related Infection Risk of Central Venous Catheter Insertion Site in ICU Patients: A Pairwise and Network Meta-Analysis. Crit Care Med 2017;45:e437-48. [Crossref] [PubMed]
- Pierce CM, Wade A, Mok Q. Heparin-bonded central venous lines reduce thrombotic and infective complications in critically ill children. Intensive Care Med 2000;26:967-72. [Crossref] [PubMed]
- Jacobs BR, Barr LL, Brilli RJ, et al. Intracatheter nitroglycerin infusion fails to prevent catheter-related venous thrombosis: a randomized, controlled trial. Intensive Care Med 2001;27:187-92. [Crossref] [PubMed]
- Anton N, Cox PN, Massicotte MP, et al. Heparin-bonded central venous catheters do not reduce thrombosis in infants with congenital heart disease: a blinded randomized, controlled trial. Pediatrics 2009;123:e453-8. [Crossref] [PubMed]
- Smith S, Dawson S, Hennessey R, et al. Maintenance of the patency of indwelling central venous catheters: is heparin necessary? Am J Pediatr Hematol Oncol 1991;13:141-3. [Crossref] [PubMed]
- Massicotte P, Julian JA, Gent M, et al. An open-label randomized controlled trial of low molecular weight heparin for the prevention of central venous line-related thrombotic complications in children: the PROTEKT trial. Thromb Res 2003;109:101-8. [Crossref] [PubMed]
- Ruud E, Holmstrøm H, De Lange C, et al. Low-dose warfarin for the prevention of central line-associated thromboses in children with malignancies--a randomized, controlled study. Acta Paediatr 2006;95:1053-9. [Crossref] [PubMed]
- Unal S, Ekici F, Cetin İİ, et al. Heparin infusion to prevent umbilical venous catheter related thrombosis in neonates. Thromb Res 2012;130:725-8. [Crossref] [PubMed]
- Shah PS, Kalyn A, Satodia P, et al. A randomized, controlled trial of heparin versus placebo infusion to prolong the usability of peripherally placed percutaneous central venous catheters (PCVCs) in neonates: the HIP (Heparin Infusion for PCVC) study. Pediatrics 2007;119:e284-91. [Crossref] [PubMed]
- Schroeder AR, Axelrod DM, Silverman NH, et al. A continuous heparin infusion does not prevent catheter-related thrombosis in infants after cardiac surgery. Pediatr Crit Care Med 2010;11:489-95. [PubMed]
- Mitchell L, Andrew M, Hanna K, et al. Trend to efficacy and safety using antithrombin concentrate in prevention of thrombosis in children receiving l-asparaginase for acute lymphoblastic leukemia. Results of the PAARKA study. Thromb Haemost 2003;90:235-44. [PubMed]
- Vidal E, Sharathkumar A, Glover J, et al. Central venous catheter-related thrombosis and thromboprophylaxis in children: a systematic review and meta-analysis. J Thromb Haemost 2014;12:1096-109. [Crossref] [PubMed]
- Esteban E, Ferrer R, Urrea M, et al. The impact of a quality improvement intervention to reduce nosocomial infections in a PICU. Pediatr Crit Care Med 2013;14:525-32. [Crossref] [PubMed]
- Zingg W, Cartier V, Inan C, et al. Hospital-wide multidisciplinary, multimodal intervention programme to reduce central venous catheter-associated bloodstream infection. PLoS One 2014;9:e93898. [Crossref] [PubMed]
- Zingg W, Imhof A, Maggiorini M, et al. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med 2009;37:2167-73. [Crossref] [PubMed]
- Salama MF, Jamal W, Al Mousa H, et al. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J Infect Public Health 2016;9:34-41. [Crossref] [PubMed]
- Cho SY, Chung DR, Ryu JG, et al. Impact of Targeted Interventions on Trends in Central Line-Associated Bloodstream Infection: A Single-Center Experience From the Republic of Korea. Crit Care Med 2017;45:e552-8. [Crossref] [PubMed]
- Furuya EY, Dick AW, Herzig CT, et al. Central Line-Associated Bloodstream Infection Reduction and Bundle Compliance in Intensive Care Units: A National Study. Infect Control Hosp Epidemiol 2016;37:805-10. [Crossref] [PubMed]
- Tang HJ, Lin HL, Lin YH, et al. The impact of central line insertion bundle on central line-associated bloodstream infection. BMC Infect Dis 2014;14:356. [Crossref] [PubMed]
- Allen GB, Miller V, Nicholas C, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line-associated bloodstream infections. Am J Infect Control 2014;42:643-8. [Crossref] [PubMed]
- O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011;52:e162. [Crossref] [PubMed]
- Ista E, van der Hoven B, Kornelisse RF, et al. Effectiveness of insertion and maintenance bundles to prevent central-line-associated bloodstream infections in critically ill patients of all ages: a systematic review and meta-analysis. Lancet Infect Dis 2016;16:724-34. [Crossref] [PubMed]
- Speroff T, Ely EW, Greevy R, et al. Quality improvement projects targeting health care-associated infections: comparing Virtual Collaborative and Toolkit approaches. J Hosp Med 2011;6:271-8. [Crossref] [PubMed]
- Marsteller JA, Sexton JB, Hsu YJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units*. Crit Care Med 2012;40:2933-9. [Crossref] [PubMed]
- Dettenkofer M, Wilson C, Gratwohl A, et al. Skin disinfection with octenidine dihydrochloride for central venous catheter site care: a double-blind, randomized, controlled trial. Clin Microbiol Infect 2010;16:600-6. [Crossref] [PubMed]
- Humar A, Ostromecki A, Direnfeld J, et al. Prospective randomized trial of 10% povidone-iodine versus 0.5% tincture of chlorhexidine as cutaneous antisepsis for prevention of central venous catheter infection. Clin Infect Dis 2000;31:1001-7. [Crossref] [PubMed]
- Langgartner J, Linde HJ, Lehn N, et al. Combined skin disinfection with chlorhexidine/propanol and aqueous povidone-iodine reduces bacterial colonisation of central venous catheters. Intensive Care Med 2004;30:1081-8. [Crossref] [PubMed]
- Levy JH, Nagle DM, Curling PE, et al. Contamination reduction during central venous catheterization. Crit Care Med 1988;16:165-7. [Crossref] [PubMed]
- Maki DG, Ringer M, Alvarado CJ. Prospective randomised trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet 1991;338:339-43. [Crossref] [PubMed]
- Mimoz O, Pieroni L, Lawrence C, et al. Prospective, randomized trial of two antiseptic solutions for prevention of central venous or arterial catheter colonization and infection in intensive care unit patients. Crit Care Med 1996;24:1818-23. [Crossref] [PubMed]
- Mimoz O, Villeminey S, Ragot S, et al. Chlorhexidine-based antiseptic solution vs alcohol-based povidone-iodine for central venous catheter care. Arch Intern Med 2007;167:2066-72. [Crossref] [PubMed]
- Vallés J, Fernández I, Alcaraz D, et al. Prospective randomized trial of 3 antiseptic solutions for prevention of catheter colonization in an intensive care unit for adult patients. Infect Control Hosp Epidemiol 2008;29:847-53. [Crossref] [PubMed]
- Yousefshahi F, Azimpour K, Boroumand MA, et al. Can a new antiseptic agent reduce the bacterial colonization rate of central venous lines in post-cardiac surgery patients? J Tehran Heart Cent 2013;8:70-5. [PubMed]
- Lai NM, Lai NA, O'Riordan E, et al. Skin antisepsis for reducing central venous catheter-related infections. Cochrane Database Syst Rev 2016;7:CD010140. [PubMed]
- García X, Sabatier C, Ferrer R, et al. Differential time to positivity of blood cultures: a valid method for diagnosing catheter-related bloodstream infections in the intensive care unit. Med Intensiva 2012;36:169-76. [Crossref] [PubMed]
- Park KH, Lee MS, Lee SO, et al. Diagnostic usefulness of differential time to positivity for catheter-related candidemia. J Clin Microbiol 2014;52:2566-72. [Crossref] [PubMed]
- Zhang Q, Wang D, Zhang W, et al. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2015;27:489-93. [The value of differential time to positivity of blood cultures in diagnosis of catheter-related bloodstream infection in patients with solid tumors in intensive care unit]. [PubMed]
- Raad I, Hanna HA, Alakech B, et al. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann Intern Med 2004;140:18-25. [Crossref] [PubMed]
Cite this article as: Zhang Z, Brusasco C, Anile A, Corradi F, Mariyaselvam M, Young P, Almog Y, Du B, Yu X, Zhu H, Zhang M, Cao Y, Hong Y. Clinical practice guidelines for the management of central venous catheter for critically ill patients. J Emerg Crit Care Med 2018;2:53.


