Effectiveness and safety of unilateral endobronchial valve applying to severe emphysema: a meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by irreversible abnormal airway inflammation, and its airflow limitation is in progress. Patients with COPD in the image are often characterized by emphysema, seriously affects the lung function. It always applied surgical lung volume reduction surgery (LVRS) in the past to reduce COPD patients’ hyperventilation, but the complications and mortality (1) can’t be ignored, even if the surgeons were very skilled. The recent bronchoscopic lung volume reduction (BLVR) surgery is considered as a good alternative for surgical LVRS. The principle of BLVR for treating patients with severe emphysema is similar as surgical LVRS by reducing emphysematous lung tissue, reducing residual volume (RV), improving the RV/total lung capacity (TLC), boosting the thoracic compliance, increasing pulmonary elastic recoil, improving the forced expiratory volume in 1 second (FEV1), FVC, improving the St. George’s Respiratory Questionnaire (SGRQ) score and exercise tolerance. In recent years, many researches using unilateral bronchial valves have been reported, most of them are case series. And a few randomized controlled trials (RCTs) were also included. A meta-analysis had reported the effectiveness and safety of BLVR (2), it used goals in minimal clinically important difference (MCID) (from baseline). We searched another two RCT studies (3,4), and we didn’t use MCID as dichotomous boundary value to make the most of data information. The effectiveness and safety of BLVR using unilateral one-way bronchial valves are also evaluated in this article in COPD patients with severe emphysema.
The study protocol was approved by the ethics committee of Zhejiang University Jinhua Hospital and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008).
Methods
Four domestic research databases, including China Biology Medicine disc, Wanfang Database, VIP Database, China National Knowledge Infrastructure, and core databases such as PubMed, EMBASE, the Cochrane Library up to January 28th 2018 were searched in our process. The initial search included 149 article abstracts. The terms “emphysema”, “valve”, “flap” were used for searching documents. Terms were modified according to each database’s index term, such as EMTREE and medical subject heading (MeSH). Two reviewers viewed all references according to the selection criteria independently. Cochrane Risk of Bias assessment tool was used to assess the quality of literature. Data from RCTs were combined to make the meta-analysis.
Inclusion/exclusion criteria
Inclusion criteria:
- COPD patients with severe emphysema;
- BLVR using one-way bronchial valve as intervention measures;
- RCT studies;
- At least one of the predetermined outcomes were reported;
- Wrote in Chinese or English.
Animal trial or nonoriginal articles such as editorials, reviews, letters, and comments were excluded. Studies with duplicate subjects or using the same outcome were also excluded. Studies of BLVR used endobronchial coils were excluded.
Outcome measures
Primary outcomes were FEV1 and RV. Secondary outcomes were 6-minute walk distance (6MWD) and quality of life including SGRQ, modified British Medical Research Council (mMRC). And the safety events: major complications (death, massive hemoptysis, pneumothorax) and minor complications (minor hemoptysis, pneumonia, COPD exacerbation, respiratory failure).
Data collection and analysis
Each step was undertaken by two researchers independently, from the literature searching to the application of selection criteria and data extraction. The predefined inclusion/exclusion criteria were used to carry out the study selection. Experts group including respiratory physicians and evidence-based medicine specialists would guide each step. Quality assessment was carried out by using the tool of the Cochrane Risk of Bias.
Data extraction and management
Two investigators extracted data independently after formulating a unanimous data extraction format by them. Continuous variables such as mean change from baseline, 95% confidence interval (CI) and standard deviation (SD) were converted according to the formula in the Cochrane Handbook for Systematic Reviews of Interventions.
Measurement and treatment effect
The mean difference (MD) and SD from each RCT were calculated, and meta-analysis was performed using fixed effect model or random effect model. Nine RCTs were finally included in the meta-analysis. Cochrane RevMan version 5.3 was used to perform the statistical analysis.
Results
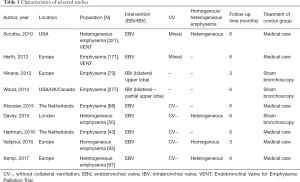
Study characteristics were shown in Table 1.
Full table
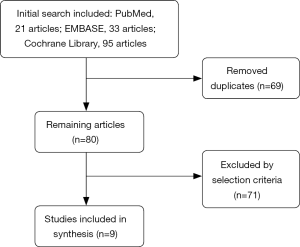
The research included PubMed 21 articles, EMBASE 33 articles, the Cochrane Library 95 articles. Our domestic databases had no RCT study, most of studies were case series or case-control studies. After screening, nine RCTs (3-11) were finally included in the meta-analysis (Figure 1), including 1,193 patients. Two articles (5,6) were using intrabronchial valve (IBV), the other 7 (3,4,7-11) used endobronchial valve (EBV). One of RCT study was excluded for its using MCID as evaluation index (12).
Assessment of risk of bias
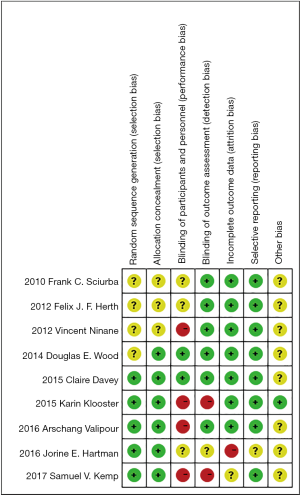
We assessed the risk of bias of these RCT studies by using Cochrane risk assessment tools (Figure 2).
Clinical effectiveness
Eight RCTs assessed effects of treatment on the patients with emphysema under unidirectional valve by comparing the differences between the treatment groups and the control groups. We conducted meta-analyses including pulmonary function (FEV1/RV), 6MWDs, SGRQ, mMRC. The results showed pulmonary function FEV1 improved in valve treatment groups, and their 6MWDs extended obviously, while the RV, SGRQ, MMRC decreased.
FEV1
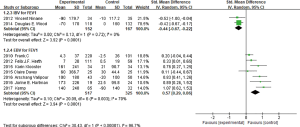
In these nine studies, two used IBV (5,6), others used EBV (3,4,7-11) in clinical researches. Among the seven studies using EBV, two studies Sciurba et al. (7) and Herth et al. (8) used FEV1 percentage for data extraction and analysis, four studies used numerical form, and one study (10) used both percentage and numerical form, meta-analysis was conducted by standardized mean difference (SMD). It showed that there was heterogeneity among seven studies (I2 =70%, P=0.003; SMD =0.57, 95% CI: 0.29–0.86) (Figure 3). The two studies and other five studies were conducted meta-analysis respectively, it showed that no significant heterogeneity between the two studies of Sciurba et al. (7) and Herth et al. (8) (I2 =0%, P=0.95; MD =6.63, 95% CI: 2.01–11.24). And there was heterogeneity among the other five studies (3,4,9-11) (I2 =49%, P=0.10), but not very remarkable, while analyzing by the fixed effect model (MD =148.16, 95% CI: 110.70–185.62), the result showed that EBV treatment group was better to improve FEV1 than the control group significantly. While there was no significant heterogeneity between the two studies using IBV (I2 =0%, P=0.81; MD =−72.46, 95% CI: −108.11 to −36.82) (Figure 3), hinted that IBV used group could reduce FEV1 than control group, namely using IBV had a harm effect on pulmonary ventilation function.
RV
In these nine studies, five studies (3,4,6,10,11) had reported the changes of the RV from baseline. It was easy to find out that Wood et al. (6) using IBV and the other four studies using EBV had obvious heterogeneity (I2 =91%). The four researches used EBV (3,4,10,11) that had reported RV were brought into meta-analysis, and heterogeneity among four studies was still remarkable (I2 =76%, P=0.006), by using the random effect model (MD =−0.54, 95% CI: −0.86 to −0.22) (Figure 4). It indicated that EBV group could reduce RV than control group, which was beneficial to the lung function of the emphysema patients. And IBV used group increased RV comparing with control group (MD =0.38, 95% CI: 0.09–0.67).
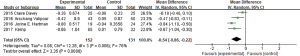
6MWD
The nine studies all had reported mean change differences of 6MWD between the BLVR treatment group and the control group from the baseline, and the meta-analysis was conducted. The result showed that these researches had obvious statistical heterogeneity (I2 =90%, P<0.00001), two of these researches used IBV, and seven other studies used EBV. When we done the heterogeneity test for these seven studies, it still showed obvious heterogeneity (I2 =86%, P<0.00001). We found that study populations in two researches (7,8) were mixed by collateral ventilation (CV+) and without collateral ventilation (CV−), while the other five selected populations were without collateral ventilation. The two studies with mixed populations showed no obvious heterogeneity (I2 =0%, P=0.37), the result was MD =14.33 (95% CI: −1.73 to 30.39), so we couldn’t think there was any statistically difference of 6MWD change between the two groups. The other five studies (3,4,9-11) without collateral ventilation showed statistical heterogeneity (I2 =85%, P<0.0001), it showed (MD =62.35, 95% CI: 32.48–92.22) by using the random effect model (Figure 5), and indicated EBV treatment group without CV could increase the score of 6MWD.
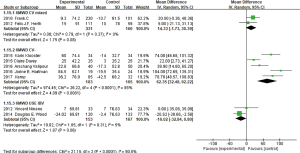
SGRQ
In the nine studies, eight of them (3-8,10,11) reported the SGRQ value changes. Two studies used IBV, the other 6 used EBV, subgroup analysis was conducted between IBV studies and EBV studies respectively. The two IBV used studies showed heterogeneity (I2 =23%, P=0.26; MD =2.70, 95% CI: −0.24 to 5.65), indicated IBV used group didn’t improve the SGRQ scores than the control group. Six studies used EBV existed certain heterogeneity (I2 =49%, P=0.08), using fixed effect model analysis (MD =−5.54, 95% CI: −7.41 to −3.67) (Figure 6). In the two studies of Sciurba et al. (7) and Herth et al. (8), populations were mixed with and without collateral ventilation who had pulmonary emphysema, and other four studies selected emphysema patients without collateral ventilation, so the subgroup analysis was made, it had no heterogeneity between the former two studies (I2 =0%, P =0.55; MD =−3.94, 95% CI: −6.40 to −1.48). And the latter four studies had some heterogeneity (I2 =46%, P=0.13; MD =−7.73, 95% CI: −10.61 to −4.86). It revealed that EBV used group comparing the control group, especially in the absence of collateral ventilation in patients with emphysema, could obviously improve the SGRQ scores.
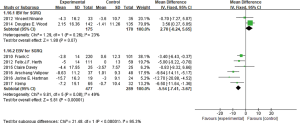
mMRC
In the nine studies, six of them (3-7,11) reported the mMRC value changes. All six studies showed certain heterogeneity (I2 =34%, P=0.18), MD =−0.33, 95% CI: −0.46 to −0.20 (Figure 7). Subgroup analysis was conducted between IBV used researches and EBV used researches. IBV used studies had no heterogeneity (I2 =0%, P=0.71; MD =−0.12, 95% CI: −0.34 to 0.10), it revealed that in the two studies used IBV, no statistical difference was found between IBV used group and control group in improving mMRC scores. While four studies using EBV (5,8-10) showed no significant heterogeneity (I2 =0%, P=0.49; MD =−0.43, 95% CI: −0.60 to −0.27), indicated EBV used group could reduce mMRC scores than the control group.
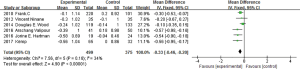
Complications
We conducted the meta-analysis of common complications in researches after imbedding one-way valves under the bronchoscope. In the nine studies, seven studies (4,6-11) reported death events caused by or relevant to one-way valve treatment under bronchoscope, the result of meta-analysis showed that the heterogeneity among seven studies was not obvious (I2 =8%, P=0.37), by using the fixed effect model and relative risk (RR) for the effect variable (RR =1.56, 95% CI: 0.67–3.60) (Figure 8), it indicated that difference of mortality between treatment group and control group had no statistical significance. Eliminating the IBV used studies, other six EBV used studies (4,7-11) had no statistical heterogeneity (I2 =0%, P=0.49), the result showed RR =1.00, 95% CI: 0.38–2.66, using fixed effect model; And the IBV used studies also suggested RR =5.70, 95% CI: 0.70–46.76. Difference of the mortality of patients between BLVR treatment group and control group had no statistical significance.
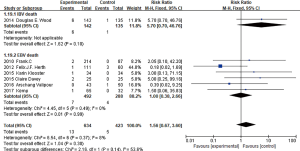
Three studies (4,7,8) reported cases of hemoptysis, and it showed no significant heterogeneity among them (I2 =0%, P=0.65; RR =5.71, 95% CI: 1.35–24.20), indicated the probability of hemoptysis between BLVR treatment group and control group had significant difference .For massive hemoptysis, only two studies(7,8)had reported, with no statistical heterogeneity between them (I2 =0%, P=0.90; RR =1.42, 95% CI: 0.15–13.48) (Figure 9), indicated that the risk of massive hemoptysis between BLVR treatment group and control group had no significant difference.
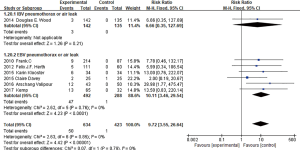
Seven studies (4,6-11) had reported pneumothorax after BLVR treatment, no significant heterogeneity among the seven studies (I2 =0%, P=0.85; RR =9.72, 95% CI: 3.55–26.64) (Figure 10), indicated BLVR treatment group raised the risk of pneumothorax than control group. When eliminating the IBV used researches, still suggested no statistical heterogeneity (I2 =0%, P=0.76; RR =10.11, 95% CI: 3.46–29.54). So, it could be thought that the BLVR treatment group increased the risk of pneumothorax than the control group.
Six studies (4,6,7,9-11) had reported the complication of pneumonia, with no statistical heterogeneity among them (I2 =0%, P=0.75; RR =1.33, 95% CI: 0.54–3.26), indicated there was no statistical difference of increasing incidence of pneumonia between BLVR treatment group and control group. After eliminating studies using IBV, the rest five studies had no statistical heterogeneity (I2 =0%, P=0.75; RR =1.60, 95% CI: 0.59–4.33), still suggested that the incidence of pneumonia between EBV and control groups had no significant difference.
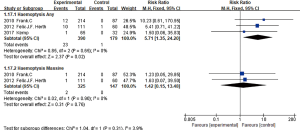
Seven studies (4,6-11) reported complication of COPD exacerbation after treatment, five studies (7-11) all used EBV mentioned COPD exacerbation requiring hospitalization, no statistical heterogeneity among these researches (I2 =0%, P=0.59; RR =1.96, 95% CI: 1.15–3.32), it showed the BLVR (EBV) treatment group increased the risk of COPD aggravating which needed hospitalization than the control group. And three (7,8,10) in these seven studies reported COPD aggravating events without hospitalization, no statistical heterogeneity among them (I2 =0%, P=0.46; RR =0.84, 95% CI: 0.63–1.13), it indicated no statistical difference of COPD exacerbation without hospitalization among the three groups.
Five studies (6-8,10,11) had reported respiratory failure, no significant heterogeneity was found among the five studies (I2 =0%, P=0.97; RR =3.54, 95% CI: 1.05–11.97), it revealed that the BLVR group had higher risk of respiratory failure than control group. But it had no significant difference between EBV group and control group (RR =2.68, 95% CI: 0.68–10.59).
So, our conclusion of safety-related outcomes is that there is statistical difference between the EBV group and control group in pneumothorax, haemoptysis, COPD exacerbation require hospitalization, the former is higher than the latter. While the difference of the probability in death, massive hemoptysis, COPD exacerbation without hospitalization, pneumonia, respiratory failure has no statistical significance.
Discussion
COPD is a chronic progressive disease, it has high disability rate and mortality, and people suffering from COPD are increasing year by year. According to epidemiological survey of northern and central areas in China in recent years, COPD population accounted for about 3% in the population aged over fifteen, and the death toll of COPD reached 1 million each year. The characteristic of COPD is irreversible damage to the lung parenchyma, and dyspnea would be caused by the destruction of dynamic mechanism and the damage of alveoli (13). Although inhalation bronchodilator and hormone drugs have certain effect, but some patients even use these drugs to the maximum dose still can’t ease dyspnea in resting state (14).
In the late 1950s, Brantigan et al. (15) was the first to propose LVRS, by removing excessive expansion of lung tissue improving the diaphragm and thoracic compensatory condition, which improved the ratio of ventilation to perfusion and relieved patients’ symptoms. In the RCTs in 2003 in the United States, it has also proved the validity of this method, but its treatment indications range was limited, it was restricted for patients with COPD who had activity limitation and the lung lesions were on the upper lobes (16). This approach still exists many adverse reactions: the mortality of postoperative is high, the mortality in 30 days after operation is 4–5%, and the surgery is expensive. In order to avoid the surgical complications, an urgent need to find a minimal invasive treatment, which can take the place of LVRS (17).
Fibrous BLVR is similar as lung volume reduction, it blocks corresponding lung segments, and makes the excessive expansion lung tissue atrophy through bronchoscope, so as to achieve the therapeutic effect of surgical LVRS. Snell et al. (18) did BLVR for 10 cases of COPD patients, significant improvement of gas exchange was found one month after operation by nuclide scan. Yim et al. (19) further confirmed the effectiveness and safety after BLVR for 2 l patients.
A large number of researches of BLVR are in progress in Europe and United States, some researches are RCTs. Some international clinical studies of BLVR such as the EBV for Emphysema Palliation Trial (VENT), IMPACT, the TRANSFORM and BeLieVeR-HIFi are famous.
Nine RCTs studies were selected in this study, including two studies using IBV, the others using EBV. Results showed that in IBV used studies, treatment group did not have obvious advantages than the control group, including lung function and activity tolerance score (6MWDs, mMRC, SGRQ). And in the studies using EBV, the experimental group had significant improvements than the control group, including pulmonary function (FEV1 and RV), and the life quality score (6MWDs, mMRC, SGRQ). And researches using EBV were all CV− patients, especially in the recent five RCT studies which showed little heterogeneity, the results indicated that EBV used group improved pulmonary function and quality of life scores significantly in patients with severe emphysema without CV, compared with control group. This might be relevant to more and more researches reported patients with CV− emphysema could benefit after using EBV, and encouraged everyone to bring CV− patients into study as the subjects. Seven studies using EBV did follow up for 3–6 months, 2 was 3 months, and the other 5 were 6 months.
In terms of safety, these nine studies showed the main complications were death, hemoptysis, pneumothorax, pneumonia, COPD exacerbation, respiratory failure, etc. The EBV treatment group increased the incidence of hemoptysis, pneumothorax, COPD exacerbation need hospitalization with statistic difference. Most of these pneumothorax patients could recover or be improved after treatment, but there were no statistical differences in the incidence of massive hemoptysis. The incidence of complications such as death, pneumonia, COPD exacerbation without hospitalization, respiratory failure between two groups has no statistically significant difference. These results indicate that the safety of using EBV are acceptable.
It is worth mentioning that Vatipour etc. (11) did the research on patients of homogenous emphysema without collateral ventilation, the results showed that EBV treatment group had significant improvements than the control group in lung function, activity tolerance and the quality of life. The VENT study in Europe (8) also confirmed that as long as the interlobar fissure was complete, the curative effect of BLVR technology was good, and highly heterogeneous emphysema was not the key to the success of treatment.
Conclusions
Our results point out that EBV treatment may be an effective and safe procedure for treating COPD patients with severe emphysema, according to existing RCT studies, but not IBV treatment. It shows that patients with complete fissure will benefit from the intervention. And the time of following up is just 3 to 6 months. Therefore, more studies are still needed to further investigate in selecting suitable patient and patient safety by long-term follow-up.
Acknowledgements
We appreciate Prof. Lorenzo Corbetta in the University of Florence and Dr. Michela Bezzi in Careggi Hospital in Italy for their guidance and teaching.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the ethics committee of Zhejiang University Jinhua Hospital and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008).
References
- Criner GJ, Cordova F, Sternberg AL, et al. The National Emphysema Treatment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med 2011;184:881-93. [Crossref] [PubMed]
- Wang Y, Lai TW, Xu F, et al. Efficacy and safety of bronchoscopic lung volume reduction therapy in patients with severe emphysema: a meta-analysis of randomized controlled trials. Oncotarget 2017;8:78031-43. [PubMed]
- Hartman JE, Klooster K, Slebos DJ, et al. Improvement of physical activity after endobronchial valve treatment in emphysema patients. Respir Med 2016;117:116-21. [Crossref] [PubMed]
- Kemp SV, Slebos DJ, Kirk A, et al. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (TRANSFORM). Am J Respir Crit Care Med 2017;196:1535-43. [Crossref] [PubMed]
- Ninane V, Geltner C, Bezzi M, et al. Multicentre European study for the treatment of advanced emphysema with bronchial valves. Eur Respir J 2012;39:1319-25. [Crossref] [PubMed]
- Wood DE, Nader DA, Springmeyer SC, et al. The IBV Valve Trial A Multicenter, Randomized, Double-Blind Trial of Endobronchial Therapy for Severe Emphysema. J Bronchology Interv Pulmonol 2014;21:288-97. [Crossref] [PubMed]
- Sciurba FC, Ernst A, Herth FJF, et al. A Randomized Study of Endobronchial Valves for Advanced Emphysema. N Engl J Med 2010;363:1233-44. [Crossref] [PubMed]
- Herth FJ, Noppen M, Valipour A, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J 2012;39:1334-42. [Crossref] [PubMed]
- Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation. N Engl J Med 2015;373:2325-35. [Crossref] [PubMed]
- Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet 2015;386:1066-73. [Crossref] [PubMed]
- Valipour A, Slebos DJ, Herth F, et al. Endobronchial Valve Therapy in Patients with Homogeneous Emphysema Results from the IMPACT Study. Am J Respir Crit Care Med 2016;194:1073-82. [Crossref] [PubMed]
- Valipour A, Herth FJ, Burghuber OC, et al. Target lobe volume reduction and COPD outcome measures after endobronchial valve therapy. Eur Respir J 2014;43:387-96. [Crossref] [PubMed]
- Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forces. Am J Respir Crit Care Med 2003;168:516-21. [Crossref] [PubMed]
- Barnes PJ. Chronic obstructive pulmonary disease * 12: New treatments for COPD. Thorax 2003;58:803-8. [Crossref] [PubMed]
- Brantigan OC, Mueller E, Kress MB. A surgical approach to pulmonary emphysema. Am Rev Respir Dis 1959;80:194-206. [PubMed]
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [Crossref] [PubMed]
- Hamacher J, Russi EW, Weder W, et al. Lung volume reduction surgery: a survey on the European experience. Chest 2000;117:1560-7. [Crossref] [PubMed]
- Snell GI, Holsworth L, Borrill ZL, et al. The potential for bronchoscopic lung volume reduction using bronchial prostheses. Chest 2003;124:1073-80. [Crossref] [PubMed]
- Yim AP, Hwong TM, Lee TW, et al. Early results of endoscopic lung volume reduction for emphysema. J Thorac Cardiovasc Surg 2004;127:1564-73. [Crossref] [PubMed]
Cite this article as: Chen WS, Zhu D, Chen H, Luo JF. Effectiveness and safety of unilateral endobronchial valve applying to severe emphysema: a meta-analysis. J Emerg Crit Care Med 2018;2:60.