Amiodarone versus digoxin for rate control in critically ill patients with rapid atrial fibrillation or flutter
Introduction
In the intensive care unit (ICU), atrial fibrillation is the most common arrhythmia and is associated with a two to fivefold increase in mortality (1). Furthermore, untreated atrial fibrillation can cause or exacerbate hypotension as well as lead to myocardial ischemia, heart failure, and organ dysfunction as a result of hypoperfusion (2,3). In the treatment of atrial fibrillation/flutter with a rapid ventricular rate, guidelines recommend to implement a rate control strategy with beta-blockers or calcium channel blockers as first line agents due to their potency and safer adverse effect profile compared to antiarrhythmics (1). In critically ill patients who may present as hemodynamically unstable, the negative inotropic and vasodilatory effects of these drugs may potentiate hypotension. Amiodarone is a commonly used antiarrhythmic in the intensive care unit and is suggested as an alternative agent for rate control by the guidelines (1). Although amiodarone is generally safe in short term use. it still may produce unfavorable effects including hypotension, bradycardia, rare acute pulmonary toxicity and drug interactions mediated by cytochrome P450 system and P-glycoprotein. Additionally long term adverse events associated with amiodarone include, thyroid abnormalities, liver dysfunction and pulmonary toxicity (4,5). In contrast to amiodarone, digoxin does not possess rhythm control properties, but instead exerts rate control effects through direct action of the atrioventricular node and by a centrally mediated vagal stimulation. Digoxin, at therapeutic doses, will increase vagal innervation, which produces a parasympathetic effect. However, in periods of high adrenergic tone (e.g., sepsis or exercise), its benefit may be decreased as vagal tone is withdrawn and sympathetic activity increases (6-8).
Literature directly comparing the two agents in a critically ill population is limited. A study from 1995 randomized patients with a HR >130 beats per minute (bpm) to receive either digoxin or amiodarone. These patients were admitted to the coronary care unit for the purpose of telemetry monitoring, however the severity of illness of these patients is unclear as the study admitted a number of patients who initially presented to an outpatient clinic. In this study, amiodarone was more effective at reducing heart rate in the first eight hours (9). These results are contrary to a 2009 study which compared the effectiveness of intravenous diltiazem to amiodarone and digoxin in patients presenting to the emergency department and found no difference in achieving target heart rate between digoxin and amiodarone. The comparison between amiodarone and digoxin, however, was a secondary outcome. Furthermore, the study included adult patients with a heart rate <120 bpm who presented to the emergency department but were otherwise stable (10).
Due to the limited and conflicting results of these studies regarding the relative efficacy of digoxin and amiodarone, clinical practice among institutions is often varied and dependent upon provider preference. The objective of this study was to elucidate whether amiodarone or digoxin is more effective for rate control in critically ill patients with atrial fibrillation or flutter.
Methods
Study design and population
This study was an IRB-approved retrospective chart review conducted at a large tertiary care academic medical center. Electronic medical records of adult patients (≥18 years) who received either intravenous digoxin or amiodarone admitted between June 1, 2014 and December 31, 2016 were reviewed. Patients were included if they had atrial fibrillation or flutter with rapid ventricular response (HR ≥110 bpm), were located in an intensive care unit (i.e., medical or surgical intensive care units or coronary care unit), and received their respective study drugs for ventricular rate control. Patients were excluded if either drug was part of their home medication regimen or if the drugs were initiated for atrial fibrillation prophylaxis. In addition, patients were excluded if the study drugs were initiated within 6 hours of each other or if the patient died within 24 hours of starting their rate control agent. Due to the nature of post-operative atrial fibrillation and the Cardiothoracic ICU (CTICU) exclusively utilizing amiodarone, patients who were located in this unit were excluded from analysis.
Data collection
Baseline demographics were collected for each patient as well as home rate control medications, left ventricular ejection fraction, and ICU and hospital length of stays. During admission to an intensive care unit, the location of the unit, reason for admission, and APACHE II scores were collected. At the time of study drug initiation, baseline heart rate and rhythm were recorded as well as any concomitant rate control medications, sedatives, or vasoactive agents. Heart rate and rhythm was recorded hourly for 24 hours. In patients receiving digoxin, the total 24-hour loading dose and the post-load serum digoxin levels were collected. In patients who received amiodarone, administration of a 150 mg bolus dose was recorded if given at the start of infusion and any subsequent bolus doses over the following 24-hour period. The total dose of amiodarone received over the 24-hour study period and duration of infusion were collected.
Primary/secondary outcomes
The primary outcome was time until ventricular rate control, defined as a heart rate <110 bpm. A subgroup analysis of the primary outcome was performed where patients in both study groups were stratified according to exogenous catecholamine use. Secondary outcomes included maintenance of target heart rate, time to sinus rhythm conversion, need for rescue therapy with alternate study drug, ICU length of stay, and 30-day mortality. Maintenance of target heart rate was defined as the percentage of time spent at goal heart rate over the 24-hour period. In addition, adverse effects, such as the incidence of bradycardia and hypotension were collected and included in the safety analysis. Hypotension was defined as either a systolic blood pressure <90 mm Hg if not on vasopressors, or a 10% increase in the infusion rate of a vasoactive medication. Bradycardia was defined as a heart rate <50 bpm.
Statistical analysis
All analyses were performed using Stata v13.1 (StataCorp LP, College Station, Texas, USA). Baseline characteristics of patients were compared using the Mann-Whitney U test and Fisher's exact test, as appropriate. The primary end point, time to ventricular rate control, as well as time to normal sinus rhythm and percentage of time with heart rate control, and length of stay endpoints were assessed using the Mann-Whitney U test. The percentage of patients with adverse events was assessed using Fisher's exact test. The primary outcome was additionally assessed using Kaplan-Meier curves and univariate Cox proportional hazard modeling, censored at 24 hours.
In order control for differences in baseline characteristics, a propensity score was calculated to determine the probability of treatment with amiodarone. The propensity score was calculated using treatment with amiodarone as the dependent variable, and all variables with a bivariate P<0.2 included as independent variables. This propensity score was then used to generate inverse probability of treatment weights (IPTW). The IPTW was then used in a weighted Cox proportional hazards model, with treatment with amiodarone serving as the sole independent variable. As left-ventricular ejection fraction (LVEF) at baseline was missing for 38 patients, the propensity score analysis was performed in the subgroup of 48 patients with complete data available (11).
Results
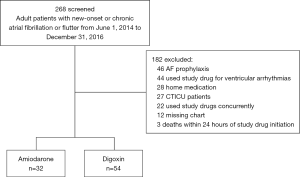
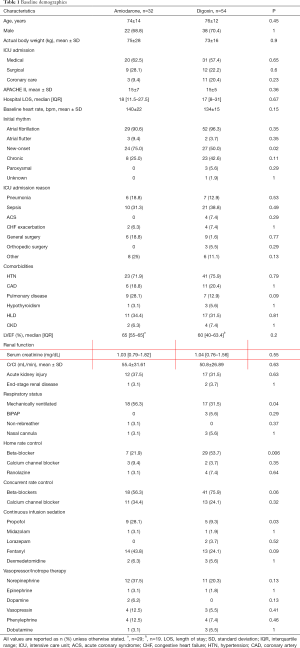
A total of 268 patients were screened for inclusion. Of those screened, 32 patients in the amiodarone group and 54 patients in the digoxin group met inclusion criteria (see Figure 1). Table 1 shows the demographic information of the study population and their baseline hemodynamic parameters. The mean age of the patients was 74 years and 69.6% were male. The mean baseline heart rate in the amiodarone group was 140 and 134 bpm (P=0.15) in digoxin group. There was no difference in the APACHE II scores between both groups. Patients in the amiodarone group were more likely to have new-onset atrial fibrillation (75% vs. 50%, P=0.02), be mechanically ventilated (56.3% vs. 31.5%, P=0.04), and started on a propofol infusion (28.1% vs. 9.3%, P=0.03). In the digoxin group, patients were more likely to be on a beta-blocker as part of their home rate control regimen (21.9% vs. 53.7%, P=0.006). The mean bolus dose of amiodarone was 154.6 mg with a total mean 24-hour dose of 933.75 mg. Amiodarone infusions were continued for a median of 36 hours and the total median 24-hour dose of digoxin was 562.5 mcg. A post-load level was obtained in 23 (42.6%) patients and was 0.93 ng/mL.
Full table
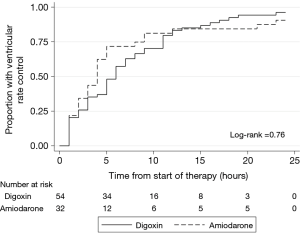
After 24 hours, rate control was achieved in 29 (90.6%) of amiodarone patients and 52 (96.3%) of the digoxin patients (P=0.35). Patients in the amiodarone group achieved target heart rate at a median of 4 hours compared with a median of 5.5 hours], P=0.46 in the digoxin group (see Table 2). Figure 2 shows the plot of the percentage of patients who achieved HR <110 bpm within the first 24 hours through Kaplan-Meier estimates. In addition, patients receiving amiodarone had persistently lower hourly heart rates compared to digoxin across all time points with a mean difference of 4±3 bpm however, the difference only reached statistical significance at hour 4 (see Figure 3).

Full table
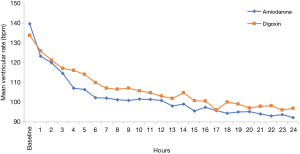
Overall, once target ventricular rate was achieved, there was no significant difference between the two agents in maintaining goal heart rate (74% vs. 78%, P=0.18). Neither amiodarone nor digoxin required rescue therapy with the alternate study drug (see Table 2). More patients in the amiodarone group were converted to normal sinus rhythm compared to the digoxin group (50% vs. 16.6%, P<0.05). There was no difference in ICU length of stay or in 30-day mortality between groups.
The frequency of hypotension was not significantly different among the two groups. One (3%) patient in the amiodarone group experienced bradycardia that required the cessation of infusion. In addition, one patient receiving amiodarone developed a mild phlebitis, which resolved upon changing the infusion site (see Table 3).

Full table
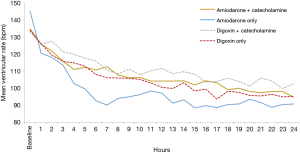
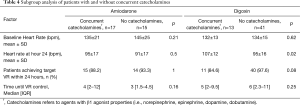
A subgroup analysis was conducted which assessed the impact of β-1 active catecholamines (e.g., norepinephrine, epinephrine, dopamine, and dobutamine) on the primary outcome (Table 4) among both amiodarone and digoxin patients. In the digoxin group, 13 patients (24%) received catecholamines concurrently, of which, 11 (84.6%) achieved target heart rate within 24 hours compared to 40 (97.6%) of patients who received digoxin without catecholamines (P=0.08). There was no significant difference in baseline heart rates between digoxin subgroups (132±13 vs. 134±15 bpm, P=0.62), however, at 24-hours the patients who received concomitant catecholamines had a significantly higher heart rate (106.5±11.59 vs. 94.49±15.53 bpm, P=0.02) (see Figure 4). In the amiodarone subgroups, there was no significant difference between the mean baseline and 24-hour heart rates. The number of patients who achieved target heart rate within 24 hours as well as the time until goal heart rate was attained was similar in both subgroups. On average, the heart rate was 7±5 bpm higher in the digoxin group compared to amiodarone in the study drug-only subgroup compared to a difference of 4±3 bpm in the concurrent study drug and catecholamine group.
Full table
Discussion
To the best of our knowledge, our study is the first directly comparing the safety and efficacy of amiodarone and digoxin in critically ill patients. In this study, there was no difference in the number of patients who achieved ventricular rate control within 24 hours, in the time until such rate control was achieved, and in the ability of the amiodarone and digoxin to maintain the rates at goal. In the subgroups of patients who received exogenous catecholamines, digoxin was unable to effectively and consistently lower the heart rate to goal—an effect not seen in the amiodarone group.
In a 2004 study by Thomas and colleagues, patients were randomized to receive sotalol, amiodarone, or digoxin (control group) with the goal of assessing the safety and efficacy of these agents in converting patients to normal sinus rhythm. This study enrolled a total of 140 patients who presented to the emergency department with symptomatic atrial fibrillation but utilized extensive exclusion criteria (i.e., uncorrectable SBP <90 mmHg, signs or symptoms of heart failure, asthma or chronic airway limitations, prolonged QTc, active hepatitis, postoperative patients, etc.). The disposition of these patients post-intervention is not addressed in the study, so the severity of illness of patients is unknown (12). Similarly, a 2009 study by Siu and colleagues compared the efficacy of intravenous diltiazem in achieving sustained ventricular rate control (defined as HR <90 bpm for ≥4 hours) to that of amiodarone and digoxin. This study also randomized stable emergency department patients with acute onset symptomatic atrial fibrillation less than 48 hours in duration and utilized extensive exclusion as well (i.e., patients with hypotension, congestive heart failure, renal failure, respiratory failure, bleeding disorders, and etc.). The disposition of these patients post-intervention is also unknown (10). Perhaps the most relevant study in terms of severity of illness is the 1995 digoxin-controlled study by Hou and colleagues which sought to assess the efficacy of amiodarone in conversion to sinus rhythm among patients with recent-onset atrial fibrillation or flutter. In this study, patients who were included for analysis were admitted to the coronary intensive care unit for telemetry monitoring. The severity of illness of these patients is unknown as a number of patients were admitted from an outpatient clinic and only 29–38% of patients received either dopamine or dobutamine, the indication for which is also unknown (9).
The findings in study are similar to the results seen by Siu and colleagues where both digoxin and amiodarone were similarly efficacious in achieving a sustained ventricular rate control (defined as HR <90 bpm for ≥4 hours); the median time to ventricular rate control was 6 hours (range: 3–15 hours) with digoxin and 7 hours (range: 1–18 hours) with amiodarone. However, an equal number of patients achieved ventricular rate control at 24 hours (74% in each group) which is similar to our study (74% vs. 78%, respectively) (10). In contrast, in the study by Hou and colleagues, patients receiving amiodarone appeared to achieve a HR <110 bpm at a mean of 2 hours compared to a mean 8 hours with digoxin. It should, however, be noted that the mean dose of amiodarone in the study was 1,383±250 mg which is higher than the dose a patient would receive in a 24-hour period today (9). This is in contrast to the 1,050 mg over 24 hours that patients would conventionally receive in clinical practice today. In the study by Thomas and colleagues found that patients achieved rate control within 30 minutes in the amiodarone group versus >6 hours in the digoxin group (12). It should be noted that in all three studies, the comparison between amiodarone and digoxin for rate control was a secondary outcome.
An interesting finding in our study was that there was no significant difference in the maintenance of target heart rate once it was achieved. This finding was unexpected due to the pharmacology of digoxin. Digoxin’s mechanism of action in heart failure is well known, though, studies have demonstrated that digoxin acts directly on the atrial tissues and the atrioventricular (AV) node. In a resting patient, digoxin enhances the parasympathetic tone of the heart via vagal nerve enervation which increases the effective and functional refractory period of the AV node (13-15). Because digoxin is predominantly a parasympathomimetic drug, it is believed to be minimally effective in states with a high sympathetic tone (e.g., sepsis) because the resultant catecholamine release can overcome digoxin’s vagotonic effects (13). Amiodarone, on the other hand, is a Vaughn-Williams class III antiarrhythmic agent which exerts its effects via potassium channel blockage, however, it has beta-blocking and calcium channel blocking effects as well and therefore does not have the same reported limitation as digoxin (16). In our study, more than half of the patients in the digoxin group were admitted to the ICU for sepsis or pneumonia—conditions which are associated with high sympathetic tone.
The most abundant data concerning the efficacy of digoxin in patients with a high sympathetic state is found in exercise and paroxysmal atrial fibrillation studies (6-8,17-20). During exercise, vagal tone is withdrawn and sympathetic activity typically predominates which renders digoxin less effective (6-8,17). Similarly, the decreased efficacy of digoxin in paroxysmal atrial fibrillation can be explained by the high catecholamine release that occurs at the onset of a paroxysm which overcomes the vagotonic effect of the drug (14,18,19).
Literature evaluating the efficacy of digoxin on rate control in sepsis is sparse. In a 1975 study, approximately 69% of clinically stable patients with atrial fibrillation (digoxin concentrations <2 ng/mL) reached a target HR <110 bpm compared to 16 clinically unstable patients who had only 39.5% of digoxin concentrations <2 ng/mL that correlated with a HR <110 bpm. This study demonstrated that significantly higher digoxin concentrations were required in the unstable patients in order to reach similar heart rate control. However, in the clinically unstable group, only two patients were septic and four patients had “pulmonary disease with hypoxemia” which was unspecified (20). Our study, however, suggests that digoxin has similar efficacy at 24 hours despite being initiated in patients with high sympathetic tone.
Studies have demonstrated that sepsis is characterized by an increased sympathetic outflow (20,21). However, in a 1995 study which compared circulating catecholamine levels in sepsis survivors and non-survivors, endogenous levels of norepinephrine, epinephrine, and vasopressin decreased to physiologic levels within 5 days in survivors (22). Perhaps, in convalescing septic patients, the increased vagal mediation of the parasympathetic tone of digoxin is able to exert its effects as endogenous catecholamine levels decline. This theory may help to explain why the mean heart rate in the digoxin group was consistently higher than in the amiodarone group across all time points, which is consistent with previous literature (10,12). However, per our subgroup analysis, it appears that digoxin group patients who received concurrent chronotropic catecholamines had both clinically and statistically higher heart rates at 24 hours compared to the digoxin patients without concurrent administration—an effect which was not observed among the amiodarone group. In the subgroup of patients not receiving concurrent catecholamines, it appears that either agent is effective at lowering the heart rate to goal <110 bpm, however patients in the digoxin group took longer by a median of 3 hours. In the subgroup of patients who require the use of exogenous catecholamines, digoxin was not as effective or consistent in lowering the heart rate to goal. Overall, critically ill patients who do not receive exogenous catecholamines appear to do well with either agent—the selection of which should depend upon patient specific factors such as expected adverse events. In this study, the use of both amiodarone and digoxin was associated with few side effects such as hypotension and bradycardia.
This study has several limitations intrinsic to the nature of a retrospective chart review. This single-center study had a small sample size. In addition, there were also differences in baseline characteristics. More patients in the amiodarone group had new-onset atrial fibrillation. This difference is likely due to amiodarone’s ability to pharmacologically convert these patients into normal sinus rhythm. In the digoxin group, more patients were on beta-blockers at home for rate control compared to the amiodarone group, however, upon admission many patients in the amiodarone group were started on beta-blockers and the difference lost statistical significance. Furthermore, as the focus of the study was acute rate control (within 24 hours), long-term safety and efficacy outcomes were not assessed.
Conclusions
In our observation, amiodarone and digoxin were had similar efficacy in the time until ventricular rate control was achieved. In addition, both groups were similarly effective in achieving and maintaining goal heart rate at 24 hours. In the subgroup of patients not receiving concurrent catecholamines, it appears that either agent is effective at lowering the heart rate to goal <110 bpm, however patients in the digoxin group take longer by a median of 3 hours. In the subgroup of patients who required the use of exogenous catecholamines, it appears that digoxin is not as effective or consistent in lowering the heart rate to goal. Overall, critically ill patients who do not receive exogenous catecholamines appear to do well with either agent—the selection of which should depend upon patient specific factors such as expected adverse events. In the safety analysis, both agents were well tolerated.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was an IRB-approved retrospective chart review conducted at a large tertiary care academic medical center.
References
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014;130:2071-104. [Crossref] [PubMed]
- Rudiger A, Harjola VP, Muller A, et al. Acute heart failure: clinical presentation, one year mortality and prognostic factors. Eur J Heart Fail 2005;7:662-70. [Crossref] [PubMed]
- Arrigo M, Bettex D, Rudiger A. Management of atrial fibrillation in critically ill patients. Crit Care Res Pract 2014;2014. [Crossref] [PubMed]
- Zimetbaum P. Amiodarone for atrial fibrillation. N Engl J Med 2007;356:935-41. [Crossref] [PubMed]
- Cushing DJ, Cooper WD, Gralinski MR, et al. The hypotensive effect of intravenous amiodarone is sustained throughout the maintenance infusion period. Clin Exp Pharmacol Physiol 2010;37:358-61. [Crossref] [PubMed]
- Beasley R, Smith DA, McHaffie DJ. Exercise heart rates at different serum digoxin concentrations in patients with atrial fibrillation. Br Med J (Clin Res Ed) 1985;290:9-11. [Crossref] [PubMed]
- Roth A, Harrison E, Mitani G, et al. Efficacy and safety of medium- and high-dose diltiazem alone and in combination with digoxin for control of heart rate at rest and during exercise in patients with chronic atrial fibrillation. Circulation 1986;73:316-24. [Crossref] [PubMed]
- Botker HE, Toft P, Klitgaard NA, et al. Influence of physical exercise on serum digoxin concentration and heart rate in patients with atrial fibrillation. Br Heart J 1991;65:337-41. [Crossref] [PubMed]
- Hou ZY, Chang MS, Chen CY, et al. Acute treatment of recent-onset atrial fibrillation and flutter with a tailored dosing regimen of intravenous amiodarone. A randomized, digoxin-controlled study. Eur Heart J 1995;16:521-8. [Crossref] [PubMed]
- Siu CW, Lau CP, Lee WL, et al. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med 2009;37:2174-9. [Crossref] [PubMed]
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424. [Crossref] [PubMed]
- Thomas SP, Guy D, Wallace E, et al. Rapid loading of sotalol or amiodarone for management of recent onset symptomatic atrial fibrillation: a randomized, digoxin-controlled trial. Am Heart J 2004;147. [Crossref] [PubMed]
- Goodman DJ, Rossen RM, Cannom DS, et al. Effect of digoxin on atrioventricular conduction. Studies in patients with and without cardiac autonomic innervation. Circulation 1975;51:251-6. [Crossref] [PubMed]
- Falk RH, Leavitt JI. Digoxin for atrial fibrillation: a drug whose time has gone? Ann Intern Med 1991;114:573-5. [Crossref] [PubMed]
- Mackstaller LL, Alpert JS. Atrial fibrillation:a review of mechanism, etiology, and therapy. Clin Cardiol 1997;20:640-50. [Crossref] [PubMed]
- Van Erven L, Schalij MJ. Amiodarone: an effective antiarrhythmic drug with unusual side effects. Heart 2010;96:1593-600. [Crossref] [PubMed]
- Rawles JM, Metcalfe MJ, Jennings K. Time of occurrence, duration, and ventricular rate of paroxysmal atrial fibrillation: the effect of digoxin. Br Heart J 1990;63:225-7. [Crossref] [PubMed]
- Gaul E, Flugelman MY, Glickson M, et al. Failure of long-term digitalization to prevent rapid ventricular response in patients with paroxysmal atrial fibrillation. Chest 1991;99:1038-40. [Crossref] [PubMed]
- Murgatroyd FD, Gibson SM, Baiyan X, et al. Double-blind placebo-controlled trial of digoxin in symptomatic paroxysmal atrial fibrillation. Circulation 1999;99:2765-70. [Crossref] [PubMed]
- Goldman S, Probst P, Selzer A, et al. Inefficacy of “therapeutic” serum levels of digoxin in controlling the ventricular rate in atrial fibrillation. Am J Cardiol 1975;35:651-5. [Crossref] [PubMed]
- Groves AC, Griffiths J, Leung F, et al. Plasma catecholamines in patients with serious postoperative infection. Ann Surg 1973;178:102-7. [Crossref] [PubMed]
- Boldt J, Menges T, Kuhn D, et al. Alterations in circulating vasoactive substances in critically ill—a comparison between survivors and non-survivors. Intensive Care Med 1995;21:218-25. [Crossref] [PubMed]
Cite this article as: Gritsenko D, Paris D, Aitken SL, Lee YI, Altshuler J. Amiodarone versus digoxin for rate control in critically ill patients with rapid atrial fibrillation or flutter. J Emerg Crit Care Med 2018;2:63.