A case of non-islet cell tumor hypoglycemia associated with pancreatic neuroendocrine tumor
Introduction
Non-islet cell tumor hypoglycemia (NICTH) represents a relatively rare occurrence, leading to episodes of severe hypoglycemia. The tumoral mass will substantially increase glucose utilization by tissues, with following low blood glucose levels. NICTH must therefore be included in the differential diagnosis of patients suffering recurrent hypoglycemia, but having no history of diabetes or any bariatric previous surgical intervention (1-4).
We report a case of NICTH with a clinical and radiological suspicion of a moderately differentiated neuroendocrine carcinoma of the pancreas (NECG2), a suspicion confirmed following surgery and open biopsy findings. Neuroendocrine carcinomas originate from malignant transformation of epithelial tissue, and when poorly differentiated (high-grade) the tumoral mass might be very aggressive with frequent metastases.
The classification of neuroendocrine tumors (NETs) has actually included different primary sites of the body such as the gastrointestinal tract, pancreas, head and neck, skin and lung. On the other hand, pancreatic neuroendocrine tumors (pNETs) represent only 1–2% of all pancreatic tumors and 7% of NETs in general, with an incidence of 0.43 per 100,000 (5,6).
Hypoglycemia might easily become an issue of emergency, with loss of conscience, confusion and profound malaise following certain low blood glucose values. Brain tissue is highly sensitive to hypoglycemia, and below a certain glycemic threshold (which seems genetic) the loss of conscience and deep coma, with probable neurologic sequelae is highly usual, if the patient survives. We have previously reported hypoglycemia following deliberate overdosing of insulin, although accidental cases are not uncommon, with a large number of oral hypoglycemic drugs imputed (7).
Case presentation
A 45-year-old female was admitted at the Service of Endocrinology at the University Hospital center ”Mother Theresa” in Tirana for the evaluation of recurrent episodes of hypoglycemia. Patient reported a two months history of bodily weakness, diaphoresis, and hunger sensation. During these episodes she obtained finger stick glucose values of 30–40 mg/dL. She did not report a history of diabetes mellitus or bariatric surgery, and was not taking any medication that could cause hypoglycemia. No underlying disease worth mentioning was suggested from her past medical history.
Her objective examination was within normal limits, except for the body mass index (BMI) of 36, 58 kg/m2. Before hospital admission a head and an abdomen CT scan were performed, with both examinations yielding no abnormalities.
During her stay in the hospital she underwent stick blood glucose monitoring which showed values as low as 22 mg/dL, clinically without severe signs of hypoglycemia. Further examinations were performed as follows: Abdominal ultrasound showed no evidence of lesions in the pancreas and liver structure; ECG-sinusal rhythm, HR—75 bpm; chest X-ray unremarkable.
The results of the laboratory findings are as follows:
- HbA1C 5.3% (reference range 4.2–6.2);
- Fasting insulinemia 17 µUI/mL (reference range 4.3–25);
- C-Peptide 0.6 nmol/L (reference range 0.35–1.17);
- Cortisolemia 163.3 ng/mL (reference range 55–230);
- Thyroid function tests were normal: TSH 1.7 µUI/mL (reference range 0.4–4.0);
- Full blood count resulted normal; liver and kidney function tests were within normal range.
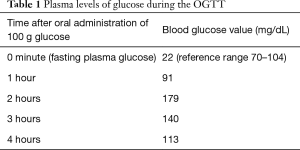
An oral glucose tolerance test (OGTT) with 100 g glucose was performed, and the results after the administration of glucose are presented in the table below (see Table 1):
Full table
During the OGTT both finger stick glucose tests and venous blood glucose tests were taken in the same moment after the oral administration of glucose and the comparison of results showed no evidence of inconsistency.
The patient underwent also the fasting test and the values of blood glucose measured are as follows:
- Blood glucose in 0 minute (fasting plasma glucose): 22 mg/dL (reference range: 70–104);
- Blood glucose after 4 hours: 113 mg/dL. This measurement was done randomly since the patient didn’t show any clinical sign of hypoglycemia.
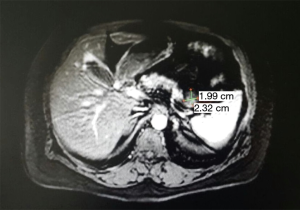
Abdomen MRI scan with intravenous contrast was performed and it showed the pancreas with normal dimensions and signal. It was evidenced a structure in the tail with dimensions 20 mm × 15 mm and the findings of MRI spoke for a NET of the pancreas tail (Figure 1).
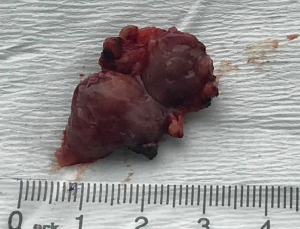
After the patient was consulted with the surgeon and anesthesiologist doctor she underwent surgery. The resection of the tumor was performed during surgery (Figure 2).
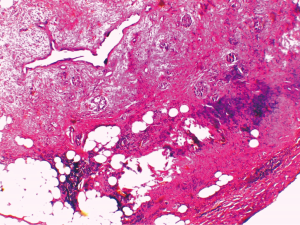
Histological examination post-surgery concluded that the formation was a moderately differentiated neuroendocrine carcinoma of the pancreas (NECG2) (Figure 3).
A blood glucose monitoring was performed the first days after surgery. The patient reported no further complaints and there were no clinical signs of hypoglycemia. All the values of blood glucose were between 80–150 mg/dL. No values of low blood glucose levels were detected.
Discussion
Different conditions might cause hypoglycemia, with drugs, multiple organ failure, endocrine diseases and hypopituitarism being more common. However, inborn errors of metabolism, autoimmune disorders and non-islet cell tumors should be considered appropriately. The treating clinician will collect a careful clinical history and perform all biochemical and diagnostic imaging tests that are deemed necessary.
Several tumors might provoke hypoglycemia, including islet and non-islet tumors. The adrenal causes of hypoglycemia were excluded in our case since the levels of cortisol were within the normal range.
A diagnostic workup for insulinoma has to be completed, with biochemical criteria including documented hypoglycemia (plasma glucose ≤55 mg/dL), inappropriately high plasma insulin ≥3 mU/mL, C-peptide ≥0.6 ng/mL (≥0.2 nmol/L), 20 pmol/L proinsulin cut-off level, and no detectable levels of hypoglycemic agents (such as metabolites of sulfonylurea) or circulating antibodies to insulin (5,8,9).
Chromogranin A (CgA) is a 49-kDa acidic glycoprotein pertaining to the granin family, a principal component of dense-core granules in neuroendocrine cells (10). The Neuroendocrine Neoplasm classification systems such as the World Health Organization classification of 2010, the European Neuroendocrine Tumor Society and the North American Neuroendocrine Tumor Society in their guidelines all agree that the immunohistochemical detection of CgA has to be performed, in order to confirm the ‘neuroendocrine’ character of tumor cell (10,11). These tumors frequently secrete IGF-2 (insulin-like growth factor 2), a hormone capable of activating the insulin receptor. This activation will initially lead to hypoglycemia, with subsequent suppression of β cell secretion, lipolysis and ketogenesis (2,5).
In the table below we are summarizing some of the recent cases published in the literature, with hypoglycemia related to a variety of tumoral origin.
As summarized in the Table 2, there is diversity of tumors that might cause hypoglycemic events, sometimes very difficult to treat, and with a final outcome largely depending on the nature and timeliness of the main tumor treatment, biological characteristic and prognosis. Obviously, the list of cases included above is merely illustrative, and a much higher number of cases are available in the literature, with sources reporting even case series rather than single case reports (14). Therefore, the necessity of considering a probable paraneoplastic underlying disorder in unexplained or unclear cases of hypoglycemia seems logical (15).
Conclusions
We report a case of severe and recurrent hypoglycemic events, with normal levels of insulinemia and C-peptide, raising the suspicion of NICTH. NICTH is a rare but serious complication of malignancies. The tumoral mass was diagnosed after an abdomen MRI, and the histological and immunohistochemical analysis were performed after the surgical removal. The expression of neuroendocrine markers such as chromogranin A and synaptophysin was confirmed. Our case highlights the necessity of taking into account the possibility of NICTH, when the laboratory and pathological data are not supporting other more frequent diagnoses, such as that of insulinoma.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Thomas J, Kumar SC. Nonislet cell tumor hypoglycemia. Case Rep Endocrinol 2013;2013. [Crossref] [PubMed]
- Khowaja A, Johnson-Rabbett B, Bantle J, et al. Hypoglycemia mediated by paraneoplastic production of Insulin like growth factor-2 from a malignant renal solitary fibrous tumor - clinical case and literature review. BMC Endocr Disord 2014;14:49. [Crossref] [PubMed]
- Scott K. Non-islet cell tumor hypoglycemia. J Pain Symptom Manage 2009;37:e1-3. [Crossref] [PubMed]
- Bodnar TW, Acevedo MJ, Pietropaolo M. Management of non-islet-cell tumor hypoglycemia: a clinical review. J Clin Endocrinol Metab 2014;99:713-22. [Crossref] [PubMed]
- Modica R, Di Sarno A, Colao A, et al. Pancreatic neuroendocrine tumor with hypoglycemia and elevated insulin-like growth factor II: a case report. J Cancer Metasta Treat 2016;2:345-7. [Crossref]
- McKenna LR, Edil BH. Update on pancreatic neuroendocrine tumors. Gland Surg 2014;3:258-75. [PubMed]
- Çakërri L, Myrtaj E, Vyshka G, et al. Prolonged Coma after Single, Unintentional Overdose of Insulin: Concurring Factors Leading to a Persistent Neurological Condition. Am J Med Case Rep 2014;2:41-3.
- Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009;94:709-28. [Crossref] [PubMed]
- Service FJ. Hypoglycemic disorders. N Engl J Med 1995;332:1144-52. [Crossref] [PubMed]
- Wang YH, Yang QC, Lin Y, et al. Chromogranin A as a marker for diagnosis, treatment, and survival in patients with gastroenteropancreatic neuroendocrine neoplasm. Medicine (Baltimore) 2014;93. [Crossref] [PubMed]
- Bosman F, Carneiro F, Hruban R, et al. editors. WHO Classification of Tumours of the Digestive System (IARC WHO Classification of Tumours) 4th Edition. 2010.
- Wong WK. Non-islet Cell Tumour Hypoglycaemia (NICTH) in Malignant Mesothelioma: Case Report. Malays J Med Sci 2015;22:81-5. [PubMed]
- Mohammedi K, Abi Khalil C, Olivier S, et al. Paraneoplastic hypoglycemia in a patient with a malignant solitary fibrous tumor. Endocrinol Diabetes Metab Case Rep 2014;2014. [Crossref] [PubMed]
- Jannin A, Espiard S, Benomar K, et al. Non-islet-cell tumour hypoglycaemia (NICTH): About a series of 6 cases. Ann Endocrinol (Paris) 2018. [Epub ahead of print].
- Efthymiou C, Spyratos D, Kontakiotis T. Endocrine paraneoplastic syndromes in lung cancer. Hormones (Athens) 2018. [Epub ahead of print].
Cite this article as: Ylli A, Husi G, Sanxhaku R, Doracaj D, Vyshka G, Cakoni R, Çakërri L. A case of non-islet cell tumor hypoglycemia associated with pancreatic neuroendocrine tumor. J Emerg Crit Care Med 2018;2:72.