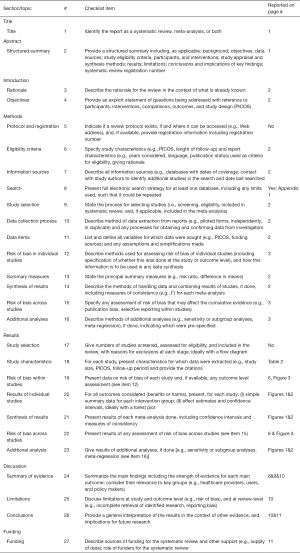
Primary decompressive craniectomy in neurocritical patients. a meta-analysis of randomized controlled trials, cohort and case-control studies
Introduction
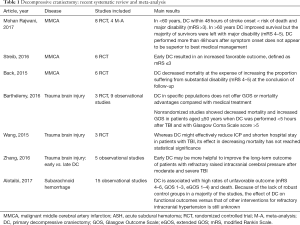
The first description on the use of decompressive craniectomy (DC) as a treatment for severe head trauma was published by Cushing in 1908, reporting a substantial reduction in the mortality of their patients (1). However, in the later decades, this treatment was not frequently used because many experts believed that the majority of survivors of DC were subsequently in a vegetative state or with severe disability (2). In the 1980s and 1990s, interest in DC was revitalized, and observational studies began to communicate good results (3,4). Virtually, all scientific literature consisting of randomized clinical trials (RCT), cohort and case-control studies on DC has been developed in the 21st century. Table 1 summarizes some of the most recent meta-analyzes and systematic reviews on this topic (5-11)
Full table
Current guidelines for different neurocritical pathologies do not yet define a clear role for this treatment (12-16). Furthermore, RCTs are still being developed in which patients randomized to the control group are not submitted to DC [acute subdural hematoma: RESCUE-ASDH (17), subarachnoid hemorrhage (SAH): NCT02995928, intracerebral hemorrhage: NCT02258919 and NCT02135783]. Nonetheless, there seems to be an increased use of this treatment (18). The ORACLE Stroke Study (19) has been recently published, a paper in which the opinion of health professionals regarding the realization of DC in malignant middle cerebral artery (MMCA) infarction was reported. Although more than half of those responding to the survey would accept DC as a life-saving treatment, 90% of respondents would agree to consider a modified Rankin Scale (mRS) =4 as an unacceptable outcome, a situation in which the patient may be unable to walk unaided in addition to requiring assistance to attend bodily needs. It is necessary to emphasize that in the Japanese registry, 92% of the survivors were in this situation or worse at 3 months of follow-up (18). These data suggest that health professionals may have an excessive expectation of the benefits that a DC provides. More importantly, mRS is a scale that is not specifically designed to assess the quality of life (QOL) of survivors.
The present systematic review and meta-analysis was performed to determine whether craniectomy is effective in improving the survival and QOL expectancy or functional status of survivors when compared to conservative treatments in the treatment of neurocritical care patients.
Methods
A systematic search was conducted using MEDLINE, PubMed, EMBASE, Cochrane Library, Google Scholar, Science Direct and Web of Science until February 10th, 2018. This review included prospective RCTs, cohort and case-control studies regarding the effects of DC on patients with severe acute intracranial pathology potentially susceptible to this surgical treatment. We used different combinations of the following search keywords: traumatic brain injury, MMCA infarction, stroke, intracranial hypertension, cerebral hemorrhage, subdural hematoma, encephalitis, cerebral venous sinus thrombosis, subarachnoid hemorrhage, intracranial pressure (ICP), craniectomy, hemicraniectomy, decompressive, and medical management (
Outcome: Our objective was to systematically analyze all articles that report on mortality and functional status of the cases at least 1 year after the insult.
The literature search was performed by two authors (J Muñoz and LC Visedo) who also compared their findings and selected articles to be reviewed by the rest of the authors. The data provided by each article were extracted and compared. Specifically, the variables determined were: number of cases, years of study development; country, number of centers participating in the study; inclusion and exclusion criteria; surgical technique of DC; medical protocol followed; functional or QOL scale with which the follow up was performed; mortality. Any discrepancies between the authors were resolved through review and consensus [
Statistical study
Original data was abstracted from each study and used to calculate the pooled odds ratio (OR) and 95% confidence interval (95% CI) in order to compare DC and non-DC groups. We used the Review Manager 5.3 (21) and EPIDAT software (22). Heterogeneity analysis was performed using Cochrane’s Q statistic and the graphic methods of Galbraith (23) and L’Abbe (24) for all the clinical trials. If the heterogeneity of each study was low or P>0.1, the fixed effect model was used (DerSimonian-Laird method); if the heterogeneity of each study was high or P<0.1, the random effects model was used. Meta-sensitivity and subgroup analysis were used to explore the sources of heterogeneity between studies. Sensitivity analysis was performed based on the leave-one-out approach.
Subgroup analysis
A separate analysis of the studies was performed according to the pathology studied (MMCA infarction, cerebral hemorrhage, subdural hematoma, encephalitis, cerebral venous sinus thrombosis, subarachnoid hemorrhage) when it was possible to detect articles of the pathologies studied.
Quality and bias evaluation
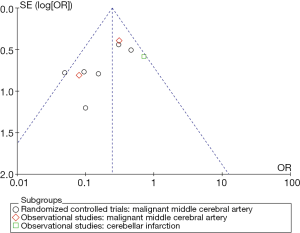
Quality control was assessed using the Jadad scale for RCTs (25), and the Newcastle-Ottawa Scale for cohort and case-control studies (26). We evaluated publication bias and small study effects visually through funnel plots and statistically using Begg and Egger tests. A P value of <0.05 was considered statistically significant. RCTs were evaluated individually to estimate the risk of bias (27).
Results
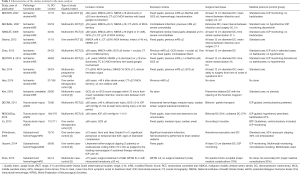
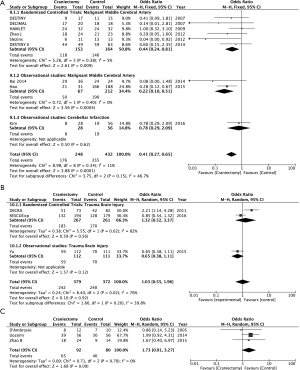
We have found 15 studies that have analyzed 1,603 patients (884 controls) (Table 2). There was no study evaluating patients with cerebral hemorrhage, subdural hematoma, encephalitis or cerebral venous sinus thrombosis at one year after the DC. Nine studies reported ischemic stroke outcomes, 6 of which were RCTs (28-33), 2 cohort studies were performed on patients with MMCA (34,35) and 1 case-control study (36) reported on posterior fossa ischemic infarction. Severe trauma brain injury (TBI) has been assessed by 2 RCTs (37,38) and a cohort study (39). Three case-control studies on DC have been published in subarachnoid hemorrhage (SAH) (40-42).
Full table
None of the studies used specific instruments to assess the QOL of the survivors. As a long-term functional evaluation criterion, 11 studies reported on the mRS and 4 the extended Glasgow Outcome Scale (eGOS). Therefore, the primary outcomes of our meta-analysis were: (I) overall mortality or vegetative state (mRS 5–6 or eGOS 1–2) and (II) mortality or moderate to severe disability on long time surveillance (mRS 4–6 or eGOS 1–4).
Patient selection
The majority of studies limit the age of indication of DC around 60 years. The DESTINY II study specifically studied older patients (33), and two observational studies on DC in SAH included patients up to 75 years of age (40,42). The patients included with MMCA stroke were cases with deterioration following an evolution of a few hours, although with moderate clinical repercussion, generally with Glasgow Coma Score (GCS) ≥6, absence of fixed pupils, and with a perfusion deficit of at least 50% of the territory of the MCA without contralateral involvement or 1/3 deficit in cerebellar infarcts. In the case of TBI, the presence of refractory intracranial hypertension was required in the two RCTs included, although in one for more than 15 minutes (≥20 mmHg, continuously or intermittently) within a 1-hour period (37) and in the other study up to 12 hours (≥25 mmHg) duration was permitted (38). In both studies even GCS =3 patients were allowed. The DECRA study included patients with reactive bilateral mydriasis (37), while the RESCUEicp excluded them (38).
Surgical technique
The standard surgical technique in the treatment of MMCA is a craniectomy of at least 12 cm in diameter. In the DECRA study (29) a larger craniectomy (a DC bifronto-parieto-occipital) was performed than that used in the RESCUEicp (30).
Medical protocol and neurocritical monitoring
In general, standard clinical practice guidelines were applied. Only two studies on MMCA infarction systematically monitored ICP (28,30), as in all the studies on patients with TBI (37,39). The case-control study of Uozomi was the only one in which multimodal monitoring techniques, including cerebral microdialysis, were systematically applied (41). Overall, barbiturates and hypothermia were excluded from treatments, with the exception of the RESCUEicp trial (38).
Outcomes
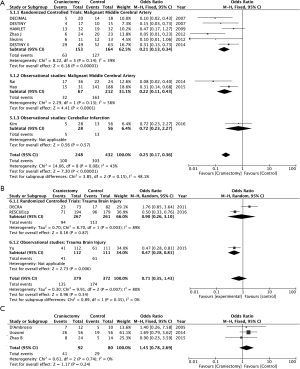
DC reduces mortality or vegetative state in MMCA stroke (OR 0.21; 95% CI, 0.14–0.32, RCT and observational studies) (Figure 1). DC also reduces the combined goal of mortality or moderate-severe disability at 12 months in MMCA stroke (OR 0.35; 95% CI, 0.21–0.58) (Figure 2). Those effects cannot be demonstrated in the treatment of refractory intracranial hypertension associated with cranial trauma (Figures 1B,2B). The sensitivity study of this meta-analysis does not establish a change in results by excluding the results of the DECRA study (37). Similarly, in SAH advantages with DC have not been found (Figures 1C,2C).
Quality and bias
Only one study reached the highest possible score (36). In general, the observational studies reached higher values than the RCTs. The small number of studies did not allow a sensitivity analysis based on methodological quality. Analysis of the funnel plot did not demonstrate the presence of publication bias (Figure 3). Table 3 summarizes the assessment of the risk of bias in selected RCTs.

Full table
Ethics
As the data is all anonymised, so that there is no chance for a person’s private medical information to leak, the ethical issues are strictly methodological. Therefore, no evaluation was requested by the Ethics Committee.
Discussion
Primary DC treatment has an indisputable rational basis: it is a question of offering the possibility of improved cerebral perfusion to patients with detrimental space occupying intracranial lesions. However, the reluctance to perform this procedure due to limitations regarding the QOL and functional situation of the survivors seems justified. Probably, the most relevant finding of our study is that Quality-Adjusted Life-Years (QALYs) and Disability-Adjusted Life-Years (DALYs) have not been applied in the analysis of the outcomes, burdens, and economic costs of DC on medium or long follow-up.
Stroke is the leading cause of disability in adults in Western society (43). Specific instruments and scales are increasingly needed to assess the outcomes of stroke treatment in a patient-centered fashion. Scales that measure neurological involvement, such as the National Institute Health Stroke Scale (NIHSS), the Scandinavian Neurological Scale of Stroke or the Canadian Scale, do not evaluate the QOL perceived by the survivors after a stroke. The mRS, the eGOS or the Barthel Index can be used to evaluate the outcomes of stroke treatment. However, these scales evaluate the physical aspects of stroke and leave aside other aspects such as memory, communication, thinking, emotions and social function (44). Some of the works published in the area of QOL and stroke have been developed with generic measures, such as the SF-36 health questionnaire (45) or the EuroQOL (46). Several QOL scales have also emerged specifically for ischemic stroke with recognized psychometric properties (47-52). The National Institutes of Health (NIH) consensus conference on the rehabilitation of persons with TBI (53) made two broad recommendations concerning QOL: (I) QOL predictors for persons with TBI, their families, and significant others should be studied, and (II) generic health-related QOL assessment instruments must be validated for use with persons with TBI, and TBI specific instruments must also be developed and validated. However, in our review only one study was identified that used specific estimates to evaluate DC. This study was not included in our meta-analysis as the follow-up was less than 1 year (54). An additional important point is that it maybe be questionable whether the so-called “retrospective consent” (the a posteriori acceptance of the functional situation) is really a demonstration of the adaptive capacity of humans (55).
The second remarkable finding from our review is the relative absence of the incorporation of advances in neuromonitoring of critically ill patients, both those submitted to DC and those treated conservatively, except for one case-control study (41). A recently published trial demonstrated a lower mortality in patients who had been treated with a strict standardized medical management protocol when compared with the medical management arms in the other trials (56). It is true that in most studies the “best medical treatment” was applied, adjusted to the current guidelines. However, although the cost-effectiveness of these practices may be under study, what is striking is the lack of use of techniques quite common in the care of neurocritical patients, such as ICP, cerebral perfusion pressure, monitoring of brain tissue oxygenation, cerebral microdialysis or EEG-derived parameters (57).
Our study indicates that a primary DC of at least 12 cm. of diameter is a technique that is indicated in certain cases of MMCA stroke. Patients ≤60 years, with unilateral infarctions ≥50% of the MCA territory, treated in the first 24–36 hours of evolution, with a good previous functional situation and that when evaluated have a GCS ≥6 and do not present bilateral fixed mydriasis, should be considered for this treatment. DC not only reduces mortality in this group of patients, but also provides the prospect of a reasonable functional outcome in the survivors. In view of the protocols followed in the MMCA stroke, the postoperative monitoring of ICP should not be considered mandatory. If we admit that the scales used for the evaluation of DC are sufficient, with the already accumulated evidence, it would not be ethical to continue carrying out studies in which the control group is deprived of that treatment in MMCA stroke. Three recent reviews, which have also included studies with a shorter follow-up period, coincide with our results (5-7) (Table 1). Mohan Rajwani et al. especially emphasize that DC performed more than 48 hours after symptom onset does not appear to be superior to best medical management (5).
The real-world indication of DC in patients suffering an ischemic stroke seems, however, to be digressing from the theoretical indications. In the Japanese registry, patients older than 60 years accounted for more than 80% of DC, 26% of patients had a GCS <6, more than half of the patients had pre-surgical signs of midbrain compression and more than one third of cases were operated on despite more than 48 hours of evolution (18). A retrospective analysis of the national database of hospitalized patients in the United States between 2005 and 2008 has shown that 28% of the patients treated with DC were over 65 years (58). Other studies have shown that there is a growing tendency to increase the age of treated patients. Bhattacharya et al. have reported that the indication DC in patients over 60 years old varied from 30.4% of all hemicraniectomies in 2001 to 36.9% in 2009 (59). The most recent trial that analyzed this question, and which included the largest number of cases, is the DESTINY II trial (33), which studied patients ≥60 years. This RCT demonstrated improved survival, but showed that only a small minority of older patients survived without disability severe enough to require assistance with most bodily needs.
According to our review, the current evidence is inconclusive and does not support the performance of primary DC in trauma or hemorrhagic injuries outside research protocols. The three observational studies published to date on hemorrhagic strokes have been excluded from our study because the follow-up period was less than 1 year (60-62). However, the results concerning mortality or severe disability at 6 months did not show any improvements over what was observed in the control group (OR 0.74; 95% CI, 0.38–1.45). Neither in severe TBI nor in SAH this surgical treatment has been shown to reduce mortality or improve functional prognosis. Even in the case of SAH, there seems to be a tendency towards a deterioration in outcome. In a recent review of 13 observational studies, Alotaibi et al. also reported similar results (11) (Table 1).
The recent publication of the results of the RESCUEicp (38) trial has once again revitalized the controversy regarding the indication of DC. In this study, the craniectomy was evaluated in patients with severe TBI and refractory intracranial hypertension. The results reflect that, although DC reduces mortality, this treatment increases the probability of vegetative state or severe disability. In addition, it is was not found to be superior to conservative treatment with regards to the rates of moderate disability or good recovery, although these authors found that a substantial number of patients with severe disability at 6 months had improved to a better outcome in 1 year. This trial found improved results when compared to those reported previously in the DECRA trial, which was also performed in the context of severe head trauma (37). These results are consistent with the fact that the current evidence does not indicate that ICP monitoring is significantly superior to no ICP monitoring in terms of the mortality of TBI patients (63). Although Zhang et al. in a review of 5 observational studies suggest a favorable effect in cases where the intervention was performed earlier (10), two recent meta-analyses confirm that it has not been possible to find benefits in mortality or prognosis of these patients in the medium or long-term follow-up (8,9) (Table 1).
On the other hand, as a limitation, our study included trials with limited simple size. Small trials are more likely to report larger beneficial effects than large trials in critical care medicine, which could be partly explained by the lower methodological quality in small trials (64).
In our opinion, many aspects remain to be clarified with respect to the indications of a DC. It is necessary to evaluate the QOL of survivors with adequate validated instruments. Probably, the common mRS and eGOS should be substituted in future studies. It is also appropriate to monitor the institutional coverage and rehabilitation services offered to survivors for the long-term evaluation of results. We also believe that it would be advisable to maximize the available battery of monitoring and treatment of neurocritical patients. It is also necessary to incorporate economic analyses of the impact of this treatment. It is, of course, essential to control that the actual clinical indications of DC conform to those that are evaluated in clinical trials. It is necessary to try to offer the society and the relatives of patients simple, straightforward and impartial information regarding these aspects. This is especially true in order to avoid the interpretation of a DC as a “life or death” dilemma in a situation of pain and strong emotional tension. It may not be time for a moratorium, but we believe it is time for a re-evaluation of this treatment strategy.
Appendix 1: Search strategies
Pathologies
Decompression OR Hemicraniectomy OR (decompres* AND (brain or crani* or surgery or surgical*)).
Craniocerebral Trauma [mh] OR Brain Edema [mh] OR Cerebrovascular Trauma [mh] OR ((head or cranial or cerebral or brain* or intra-cranial or inter-cranial) OR (“diffuse axonal injury” OR “diffuse axonal injuries”) OR Stroke [mh] OR Stroke [tiab] ischemic stroke [mh] Or stroke [tiab] OR brain hemorrahag* [mh] or brain hemorrahag* [tiab] OR cerebral hemorrahag* [mh] or cerebral hemorrhag* [tiab] OR brain haemorrahag* [mh] or brain haemorrahag* [tiab] OR cerebral haemorrahag* [mh] or cerebral haemorrhag* [tiab] OR subarachnoid hemorrhag* [mh] OR subarachnoid hemorrhage* [tiab] OR subarachnoid haemorrhag* [mh] OR subarachnoid haemorrhage [tiab].
Prognosis
Glasgow Coma Score [mh] OR Glasgow Coma Score [tiab] OR GOS [mh] OR GOS [tiab] OR modified Rankin Scale OR mRH OR (“persistent vegetative state”) OR ((unconscious* OR coma* OR concuss*).
Publications
Randomized controlled trials, cohorts and case controls studies
(clinical[Title/Abstract] AND trial[Title/Abstract]) OR clinical trials as topic[MeSH Terms] OR (clinical[Title/Abstract] AND trial[Title/Abstract]) OR random allocation[MeSH Terms] OR RCT OR therapeutic use[MeSH Subheading] OR MH random assignment OR TX randomised OR randomized OR randomly OR random order OR random sequence or randomly allocated or at random OR exp cohort studies/ OR cohort$.tw. OR controlled clinical trial.pt. OR epidemiologic methods/ OR exp case-control studies/OR(case$ and control$).tw. OR exp cohort analysis OR exp longitudinal study/ OR exp prospective study/ OR exp follow up/ OR cohort$.tw. OR exp case control study/ OR (case$ and control$).tw.
Specific Medline review and meta-analysis
1. Meta-Analysis as Topic; 2. meta analy$.tw.; 3. metaanaly$.tw.; 4. Meta-Analysis/; 5. (systematic adj (review$1 or overview$1)).tw.; 6. exp Review Literature as Topic/; 7. or/1-6; 8. cochrane.ab.; 9. embase.ab.; 10. (psychlit or psyclit).ab.; 11. (psychinfo or psycinfo).ab.; 12. (cinahl or cinhal).ab.; 13. science citation index.ab.; 14. bids.ab.; 15. cancerlit.ab.; 16. or/8-15; 17. reference list$.ab.; 18. bibliograph$.ab.; 19. hand-search$.ab.; 20. relevant journals.ab.; 21. manual search$.ab.; 22. or/17-21; 23. selection criteria.ab.; 24. data extraction.ab.; 25. 23 or 24; 26. Review/; 27. 25 and 26; 28. Comment/; 29. Letter/; 30. Editorial/; 31. animal/; 32. human/; 33. 31 not (31 and 32); 34. or/28-30,33; 35. 7 or 16 or 22 or 27; 36. 35 not 34.
Specific Embase review and meta-analysis
1. exp Meta Analysis/; 2. ((meta adj analy$) or metaanalys$).tw.; 3. (systematic adj (review$1 or overview$1)).tw.; 4. or/1-3; 5. cancerlit.ab.; 6. cochrane.ab.; 7. embase.ab.; 8. (psychlit or psyclit).ab.; 9. (psychinfo or psycinfo).ab.; 10. (cinahl or cinhal).ab.; 11. science citation index.ab.; 12. bids.ab.; 13. or/5-12; 14. reference lists.ab.; 15. bibliograph$.ab.; 16. hand-search$.ab.; 17. manual search$.ab.; 18. relevant journals.ab.; 19. or/14-18; 20. Data extraction.ab.; 21. selection criteria.ab.; 22. 20 or 21; 23. review.pt.; 24. 22 and 23; 25. letter.pt.; 26. editorial.pt.; 27. animal/; 28. human/; 29. 27 not (27 and 28); 30. or/25-26,29; 31. 4 or 13 or 19 or 24; 32. 31 not 30.
Specific CINAHL review and meta-analysis
1. Meta analysis/; 2. Meta analys$.tw.; 3. Metaanaly$.tw.; 4. exp Literature review/; 5. (systematic adj (review or overview)).tw.; 6. Or/1-5; 7. Commentary.pt.; 8. Letter.pt.; 9. Editorial.pt.; 10. Animals/; 11 Or/7-10; 12. 6 not 11.
Appendix 2: Checklist of PRISMA Guidelines for meta-analysis
Acknowledgements
We are immensely grateful to Juan Ignacio Nuñez, PhD, for their technical assistance in the edition of this paper.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cushing H. Subtemporal Decompressive Operations for the Intracranial Complications Associated with Bursting Fractures of the Skull. Ann Surg 1908;47:641-644.1. [Crossref] [PubMed]
- Kolias AG, Kirkpatrick PJ, Hutchinson PJ. Decompressive craniectomy: past, present and future. Nat Rev Neurol 2013;9:405-15. [Crossref] [PubMed]
- Polin RS, Shaffrey ME, Bogaev CA, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery 1997;41:84-92. [Crossref] [PubMed]
- Whitfield PC, Patel H, Hutchinson PJ, et al. Bifrontal decompressive craniectomy in the management of posttraumatic intracranial hypertension. Br J Neurosurg 2001;15:500-7. [Crossref] [PubMed]
- Mohan Rajwani K, Crocker M, Moynihan B. Decompressive craniectomy for the treatment of malignant middle cerebral artery infarction. Br J Neurosurg 2017;31:401-9. [Crossref] [PubMed]
- Streib CD, Hartman LM, Molyneaux BJ. Early decompressive craniectomy for malignant cerebral infarction: Meta-analysis and clinical decision algorithm. Neurol Clin Pract 2016;6:433-43. [Crossref] [PubMed]
- Back L, Nagaraja V, Kapur A, et al. Role of decompressive hemicraniectomy in extensive middle cerebral artery strokes: a meta-analysis of randomised trials. Intern Med J 2015;45:711-7. [Crossref] [PubMed]
- Barthélemy EJ, Melis M, Gordon E, et al. Decompressive Craniectomy for Severe Traumatic Brain Injury: A Systematic Review. World Neurosurg 2016;88:411-20. [Crossref] [PubMed]
- Wang R, Li M, Gao WW, et al. Outcomes of Early Decompressive Craniectomy Versus Conventional Medical Management After Severe Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2015;94. [Crossref] [PubMed]
- Zhang K, Jiang W, Ma T, et al. Comparison of early and late decompressive craniectomy on the long-term outcome in patients with moderate and severe traumatic brain injury: a meta-analysis. Br J Neurosurg 2016;30:251-7. [Crossref] [PubMed]
- Alotaibi NM, Elkarim GA, Samuel N, et al. Effects of decompressive craniectomy on functional outcomes and death in poor-grade aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurosurg 2017;127:1315-25. [Crossref] [PubMed]
- Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46:2032-60. [Crossref] [PubMed]
- Carney N, Totten AM, O’Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017;80:6-15.
- Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870-947. [Crossref] [PubMed]
- Steiner T, Juvela S, Unterberg A, et al. European Stroke Organization. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis 2013;35:93-112. [Crossref] [PubMed]
- Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012;43:1711-37. [Crossref] [PubMed]
- Protocol 14PRT/6944: Randomised Evaluation of Surgery with Craniectomy for patients Undergoing Evacuation of Acute Subdural Haematoma (RESCUE-ASDH) - ISRCTN87370545. 2015. Accessed January 3, 2017. Available online: https://www.thelancet.com/protocol-reviews/14PRT-6944
- Suyama K, Horie N, Hayashi K, et al. Nationwide survey of decompressive hemicraniectomy for malignant middle cerebral artery infarction in Japan. World Neurosurg 2014;82:1158-63. [Crossref] [PubMed]
- Honeybul S, Ho KM, Blacker DW, et al. ORACLE Stroke Study: Opinion Regarding Acceptable Outcome Following Decompressive Hemicraniectomy for Ischemic Stroke. Neurosurgery 2016;79:231-6. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6. [Crossref] [PubMed]
- Review Manager (RevMan) (Computer program). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Epidat (computer program). Version 4.2. Xunta de Galicia, Conselleria de Sanidade, 2016. Available online: http://dxsp.sergas.es/ApliEdatos/Epidat/cas/default.asp
- Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med 1988;7:889-94. [Crossref] [PubMed]
- L’Abbé KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Ann Intern Med 1987;107:224-33. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. Accessed February 4, 2017. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Higgins JP, Altman DG, Gotzche P, et al. The Cochrane Collaboration’s tool for assessing of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Jüttler E, Schwab S, Schmiedek P, et al. Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY): a randomized, controlled trial. Stroke 2007;38:2518-25. [Crossref] [PubMed]
- Vahedi K, Vicaut E, Mateo J, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL trial). Stroke 2007;38:2506-17. [Crossref] [PubMed]
- Hofmeijer J, Kappelle LJ, Algra A, et al. Surgical decompression for space occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial (HAMLET)): a multicentre, open, randomised trial. Lancet Neurol 2009;8:326-33. [Crossref] [PubMed]
- Slezins J, Keris V, Bricis R, et al. Preliminary results of randomized controlled study on decompressive craniectomy in treatment of malignant middle cerebral artery stroke. Medicina (Kaunas) 2012;48:521-4. [PubMed]
- Zhao J, Su YY, Zhang Y, et al. Decompressive hemicraniectomy in malignant middle cerebral artery infarct: a randomized controlled trial enrolling patients up to 80 years old. Neurocrit Care 2012;17:161-71. [Crossref] [PubMed]
- Jüttler E, Unterberg A, Woitzik J, et al. Hemicraniectomy in Older Patients with Extensive Middle-Cerebral-Artery Stroke (DESTINY II). N Engl J Med 2014;370:1091-100. [Crossref] [PubMed]
- Rai VK, Bhatia R, Prasad K, et al. Long-term outcome of decompressive hemicraniectomy in patients with malignant middle cerebral artery infarction: a prospective observational study. Neurol India 2014;62:26-31. [Crossref] [PubMed]
- Hao Z, Chang X, Zhou H, et al. A Cohort Study of Decompressive Craniectomy for Malignant Middle Cerebral Artery Infarction: A Real-World Experience in Clinical Practice. Medicine (Baltimore) 2015;94. [Crossref] [PubMed]
- Kim MJ, Park SK, Song J, et al. Preventive Suboccipital Decompressive Craniectomy for Cerebellar Infarction: A Retrospective-Matched Case-Control Study. Stroke 2016;47:2565-73. [Crossref] [PubMed]
- Cooper DJ, Rosenfeld JV, Murray L, et al. Decompressive craniectomy in diffuse traumatic brain injury (DECRA). N Engl J Med 2011;364:1493-502. [Crossref] [PubMed]
- Hutchinson PJ, Kolias AG, Timofeev IS, et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension (RESCUEicp). N Engl J Med 2016;375:1119-30. [Crossref] [PubMed]
- Yu P, Tian Q, Wen X, et al. Analysis of Long-Term Prognosis and Prognostic Predictors in Severe Brain Injury Patients Undergoing Decompressive Craniectomy and Standard Care. J Craniofac Surg 2015;26:e635-41. [Crossref] [PubMed]
- D’Ambrosio AL, Sughrue ME, Yorgason JG, et al. Decompressive hemicraniectomy for poor-grade aneurysmal subarachnoid hemorrhage patients with associated intracerebral hemorrhage: clinical outcome and quality of life assessment. Neurosurgery 2005;56:12-9; dicussion 19-20.
- Uozumi Y, Sakowitz O, Orakcioglu B, et al. Decompressive craniectomy in patients with aneurysmal subarachnoid hemorrhage: a single-center matched-pair analysis. Cerebrovasc Dis 2014;37:109-15. [Crossref] [PubMed]
- Zhao B, Zhao Y, Tan X, et al. Primary decompressive craniectomy for poor-grade middle cerebral artery aneurysms with associated intracerebral hemorrhage. Clin Neurol Neurosurg 2015;133:1-5. [Crossref] [PubMed]
- Bonita R, Solomon N, Broad J. Prevalence of stroke and stroke-related disability. Estimates from the Auckland Stroke Studies. Stroke 1997;28:1898-902. [Crossref] [PubMed]
- De Haan R, Horn J, Limburg M, et al. A comparison of five stroke scales with measures of disability, handicap, and quality of life. Stroke 1993;24:1178-81. [Crossref] [PubMed]
- Dorman PJ, Slattery J, Farrell B, et al. A randomised comparison of the EuroQol and Short Form-36 after stroke. BMJ 1997;315:461. [Crossref] [PubMed]
- Golicki D, Niewada M, Buczek J, et al. Validity of EQ-5D-5L in stroke. Qual Life Res 2015;24:845-50. [Crossref] [PubMed]
- van Straten A, de Haan RJ, Limburg M, et al. A stroke-adapted 30-item version of the Sickness Impact Profile to assess quality of life (SA-SIP30). Stroke 1997;28:2155-61. [Crossref] [PubMed]
- Duncan PW, Wallace D, Lai SM, et al. The Stroke Impact Scale Version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke 1999;30:2131-40. [Crossref] [PubMed]
- Williams LS, Weinberger M, Harris LE, et al. Development of a stroke-specific quality of life scale. Stroke 1999;30:1362-9. [Crossref] [PubMed]
- Doyle PJ. Measuring health outcomes in stroke survivors. Arch Phys Med Rehabil 2002;83:S39-43. [Crossref] [PubMed]
- Hilari K, Byng S, Lamping DL, et al. Stroke and aphasia quality of life scale-39 (SAQOL-39). Evaluation of acceptability, reliability, and validity. Stroke 2003;34:1944-50. [Crossref] [PubMed]
- Buck D, Jacoby A, Massey A, et al. Development and validation of NEWSQOL, the Newcastle stroke-specific quality of life measure. Cerebrovasc Dis 2004;17:143-52. [Crossref] [PubMed]
- Consensus conference. Rehabilitation of persons with traumatic brain injury. NIH Consensus Development Panel on Rehabilitation of Persons with Traumatic Brain Injury. JAMA 1999;282:974-83. [Crossref] [PubMed]
- Taylor A, Butt W, Rosenfeld J, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst 2001;17:154-62. [Crossref] [PubMed]
- Honeybul S, Ho KM, Gillett G. Outcome following decompressive hemicraniectomy for malignant cerebral infarction: ethical considerations. Stroke 2015;46:2695-98. [Crossref] [PubMed]
- Frank JI, Schumm LP, Wroblewski K, et al. Hemicraniectomy and Durotomy Upon Deterioration From Infarction-Related Swelling Trial (HeADDFIRST): A Randomized Pilot Clinical Trial. Stroke 2014;45:781-7. [Crossref] [PubMed]
- Citerio G, Oddo M, Taccone FS. Recommendations for the use of multimodal monitoring in the neurointensive care unit. Curr Opin Crit Care 2015;21:113-9. [Crossref] [PubMed]
- Adeoye O, Hornung R, Khatri P, et al. The rate of hemicraniectomy for acute ischemic stroke is increasing in the United Sates. J Stroke Cerebrovasc Dis 2011;20:251-4. [Crossref] [PubMed]
- Bhattacharya P, Kansara A, Chaturvedi S, et al. What drives the increasing utilization of hemicraniectomy in acute ischaemic stroke? J Neurol Neurosurg Psychiatry 2013;84:727-31. [Crossref] [PubMed]
- Maira G, Anile C, Colosimo C, et al. Surgical treatment of primary supratentorial intracerebral hemorrhage in stuporous and comatose patients. Neurol Res 2002;24:54-60. [Crossref] [PubMed]
- Ma L, Liu WG, Sheng HS, et al. Decompressive craniectomy in addition to hematoma evacuation improves mortality of patients with spontaneous basal ganglia hemorrhage. J Stroke Cerebrovasc Dis 2010;19:294-8. [Crossref] [PubMed]
- Fung C, Murek M. Decompressive hemicraniectomy in patients with supratentorial intracerebral hemorrhage. Stroke 2012;43:3207-11. [Crossref] [PubMed]
- Yuan Q, Wu X, Sun Y, et al. Impact of intracranial pressure monitoring on mortality in patients with traumatic brain injury: a systematic review and meta-analysis. J Neurosurg 2015;122:574-87. [Crossref] [PubMed]
- Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Critical Care 2013;17:R2. [Crossref] [PubMed]
Cite this article as: Muñoz J, Keough EA, Barrios JC, Fernández NJ, Dalorzo MG, Visedo LC. Primary decompressive craniectomy in neurocritical patients. a meta-analysis of randomized controlled trials, cohort and case-control studies. J Emerg Crit Care Med 2018;2:73.