
Blood purification in critically ill patients with sepsis and septic shock
Challenge posed by increase in elderly patients with sepsis and septic shock
Although several studies have obtained negative or controversial results regarding blood purification (1,2), such results need to be interpreted carefully and intensivists should prepare for the challenge posed by increases in the percentage of adults aged older than 65 years. According to a 2017 World Bank report, the overall percentage of people older than 65 years is 27% in Japan, 23% in Italy, and 21% in Germany. Overall mortality is high in elderly patients with sepsis, and in-hospital mortality reported in patients aged 65 is approximately 30−60%, rising to 40−80% in those aged 80 years and older (3). Functional impairment in both mediated immunity and humoral immune responses and dysfunction of the liver and kidney has been noted in elderly patients with sepsis and septic shock. Moreover, elderly patients with sepsis and septic shock may be more susceptible to endotoxin storm, cytokine storm, subsequent endothelial dysfunction, and eventual multiorgan dysfunction. Removal of endotoxins, cytokines, and toxic mediators may play a crucial role in reducing endothelial and multiorgan dysfunction.
Pathophysiology of sepsis and mechanisms of blood purification
Sepsis is a combination of physiological, pathological, and biochemical abnormalities induced by infection (4). A dysregulated host response to infection may result in life-threatening multi-organ dysfunction. Patients with sepsis first experience an early phase resulting from massive and deregulated activation of innate and adaptive immunity, which is followed by a second late phase caused by immunosuppression and lymphocyte exhaustion (5). Therefore, the primary goal of blood purification is to attenuate the overwhelming systemic inflammation and subsequent immunosuppression. The additional benefits of blood purification include blood detoxification, acid–base control, fluid and electrolyte balance, and improvement of encephalopathy, lung edema, and bone marrow suppression. Blood purification therapies are designed to remove the following substances from blood circulation: microbial toxins, inflammatory mediators, and toxic metabolites. Microbial toxins include endotoxins, exotoxins, fungal toxins, and viral toxins. Cytokine storm results from the following inflammatory mediators: tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), IL-6, IL-8, and IL-10. Toxic metabolites include uremic toxins, amino acid derivatives, and acidic and vasogenic substances. The mechanisms of blood purification include hemodialysis, hemofiltration, hemodiafiltration, plasmapheresis, coupled plasma filtration adsorption, plasma exchange, and hemoadsorption/hemoperfusion.
Clinical research on mainstream blood purification
Endotoxin hemoadsorption
Polymyxin B is a cationic polypeptide antibiotic with high affinity to bind and neutralize endotoxin, and endotoxin can be removed through hemoadsorption with a cartridge with immobilized adsorbent of polymyxin B. In 2009, Cruz et al. revealed a reduction of mortality and improvement of hemodynamics and pulmonary oxygenation through polymyxin B hemoperfusion (PMX-HP) (6). However, a propensity-matched analysis in 2014 (7), a multicenter randomized control trial in 2015 (8), and a systemic review and meta-analysis in 2018 (1) all reported an absence of survival benefits. Regarding our clinical experiences of PMX-HP, our retrospective multivariate regression analysis revealed that PMX-HP was associated with a lower 28-day mortality [odds ratio: 0.18; 95% confidence interval (CI): 0.04−0.92] (9). Furthermore, in the disease severity subgroup meta-analysis in our systemic review, we showed a significant reduction of mortality in the intermediate-risk group (risk ratio: 0.84; 95% CI: 0.77−0.92) and high-risk group (risk ratio: 0.64; 95% CI: 0.52−0.78) (10). In addition, our nonlinear meta-regression with restricted cubic spline revealed an inverse association between baseline mortality and a reduction in the risk of mortality (10). In our clinical experience of PMX-HP, to obtain clinical benefits from PMX-HP, the following are recommended: selection of the appropriate patients, initiation of PMX-HP at the correct time, appropriate protocol of anticoagulant use (11), and ensuring multidisciplinary collaboration for sepsis and septic shock management.
Cytokine hemoadsorption
A highly adsorptive and biocompatible polymer (CytoSorb) has been designed to remove multiple inflammatory mediators from the bloodstream in a size range of approximately 10−55 kD. A small case series revealed that CytoSorb resulted in rapid hemodynamic stabilization and increased survival, particularly in patients for whom therapy was started within 24 h (12). A combination of AN69-based membrane hemofilter and surface treatment with polyethyleneimine (oXiris) is designed to remove endotoxins and cytokines from blood circulation. A case series demonstrated that oXiris treatment reduced IL-6 level and improved hemodynamics (13). Coupled plasma filtration adsorption (CPFA) is another technique for removing inflammatory mediators. Three studies have indicated that CPFA improved hemodynamics in patients with sepsis and septic shock (14-16), but no survival benefit was observed. Further multi-center randomized control trials are therefore required to investigate the survival and long-term clinical benefits resulting from CytoSorb, oXiris, and CPFA among patients with sepsis and septic shock.
High cut-off hemodialysis/hemofiltration
During high cut-off hemodialysis/hemofiltration, the pore size of high cut-off membrane is increased from 0.01 to 0.02 µm, and this increases the removal of inflammatory mediators. A small-scaled study found that high cut-off hemodialysis/hemofiltration reduced requirement of vasopressors and was superior to conventional hemofiltration in eliminating IL-6 from the bloodstream (17). One detrimental effect of high cut-off membrane is the loss of albumin during hemofiltration (18). Further studies are required to investigate the balance between beneficial and detrimental effects.
Future perspective
More novel devices, filters, and cartridges are being designed to meet clinical requirement. Moreover, a blood-cleaning device is under development to capture a broad range of pathogens and toxin by using magnetic nanobeads (19). Magnets pull the nanobead-bound pathogens and toxins from the blood without leaving microbial toxins in the bloodstream and without activating complement factors or coagulation. Extracorporeal removal of pathogens from the blood is a promising treatment for severe bacteremia in elderly patients with sepsis and septic shock. If most bacteria can be removed from the blood of such patients before the administration of antibiotics, fewer microbial toxins will be produced in the blood, and less systemic inflammation will be activated. Furthermore, randomized control trials must be conducted in an appropriate and careful manner. In addition to short-term survival benefits, we should carefully investigate the preservation of organ function, long-term clinical benefits, and long-term survival. To conduct an appropriate randomized control trial in the future, we must learn advanced designs of clinical trials, including factorial trials, adaptive trials, and platform trials. A clinical trial with an advanced design may reduce trial cost and hasten completion. We suggest that numerous potential components be considered to obtain clinical benefits from blood purification in patients with sepsis and septic shock (Table 1).
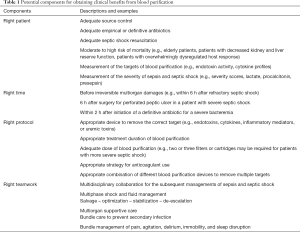
Full table
Conclusions
Although blood purification is theoretically to improve clinical outcomes, no consensus has been reached regarding the optimal timing and optimal devices, filters, or cartridges for patients with sepsis and septic shock. To face the challenge posed by increase in elderly patients with sepsis and septic shock, further randomized control trials with advanced designs are necessary to investigate the survival and clinical benefits of blood purification.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fujii T, Ganeko R, Kataoka Y, et al. Polymyxin B-immobilized hemoperfusion and mortality in critically ill adult patients with sepsis/septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 2018;44:167-78. [Crossref] [PubMed]
- Zhou F, Peng Z, Murugan R, et al. Blood purification and mortality in sepsis: a meta-analysis of randomized trials. Crit Care Med 2013;41:2209-20. [Crossref] [PubMed]
- Rowe TA, McKoy JM. Sepsis in Older Adults. Infect Dis Clin North Am 2017;31:731-42. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011;306:2594-605. [Crossref] [PubMed]
- Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA 2009;301:2445-52. [Crossref] [PubMed]
- Iwagami M, Yasunaga H, Doi K, et al. Postoperative polymyxin B hemoperfusion and mortality in patients with abdominal septic shock: a propensity-matched analysis. Crit Care Med 2014;42:1187-93. [Crossref] [PubMed]
- Payen DM, Guilhot J, Launey Y, et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med 2015;41:975-84. [Crossref] [PubMed]
- Lee CT, Tu YK, Yeh YC, et al. Effects of polymyxin B hemoperfusion on hemodynamics and prognosis in septic shock patients. J Crit Care 2018;43:202-6. [Crossref] [PubMed]
- Chang T, Tu YK, Lee CT, et al. Effects of Polymyxin B Hemoperfusion on Mortality in Patients With Severe Sepsis and Septic Shock: A Systemic Review, Meta-Analysis Update, and Disease Severity Subgroup Meta-Analysis. Crit Care Med 2017;45:e858-64. [Crossref] [PubMed]
- Lee CT, Chao A, Lai CH, et al. Heparin dosing score protocol for anticoagulation during polymyxin B hemoperfusion. J Formos Med Assoc 2018;117:652-3. [Crossref] [PubMed]
- Kogelmann K, Jarczak D, Scheller M, et al. Hemoadsorption by CytoSorb in septic patients: a case series. Crit Care 2017;21:74. [Crossref] [PubMed]
- Turani F CF, Barchetta R, et al. Continuous renal replacement therapy with the adsorbent membrane oXiris in septic patients: a clinical experience. Crit Care 2013;17:63. [Crossref]
- Berlot G, Agbedjro A, Tomasini A, et al. Effects of the volume of processed plasma on the outcome, arterial pressure and blood procalcitonin levels in patients with severe sepsis and septic shock treated with coupled plasma filtration and adsorption. Blood Purif 2014;37:146-51. [Crossref] [PubMed]
- Livigni S, Bertolini G, Rossi C, et al. Efficacy of coupled plasma filtration adsorption (CPFA) in patients with septic shock: a multicenter randomised controlled clinical trial. BMJ Open 2014;4. [Crossref] [PubMed]
- Franchi M, Giacalone M, Traupe I, et al. Coupled plasma filtration adsorption improves hemodynamics in septic shock. J Crit Care 2016;33:100-5. [Crossref] [PubMed]
- Morgera S, Haase M, Kuss T, et al. Pilot study on the effects of high cutoff hemofiltration on the need for norepinephrine in septic patients with acute renal failure. Crit Care Med 2006;34:2099-104. [Crossref] [PubMed]
- Ricci Z, Romagnoli S, Ronco C. High cut-off membranes in acute kidney injury and continuous renal replacement therapy. Int J Artif Organs 2017;40:657-64. [Crossref] [PubMed]
- Kang JH, Super M, Yung CW, et al. An extracorporeal blood-cleansing device for sepsis therapy. Nat Med 2014;20:1211-6. [Crossref] [PubMed]
Cite this article as: Chiu CT, Yeh YC. Blood purification in critically ill patients with sepsis and septic shock. J Emerg Crit Care Med 2018;2:77.


