
A potential diagnostic protocol for critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients
Introduction
Critical illness-related corticosteroid insufficiency (CIRCI) is defined as inadequate cellular corticosteroid activity for the severity of patient’s critical illness (1). Physicians still lack effective clinical tools to diagnose CIRCI, which manifests by insufficient glucocorticoid (GC)-glucocorticoid receptor (GR)-mediated down-regulation of proinflammatory cytokines (2). The 2008 guidelines recommend that the diagnosis should be an increase in total cortisol at 60 min from baseline <9 µg/dL after a 250-µg cosyntropin stimulation test or a random total cortisol <10 µg/dL (1). Considering only one single-center unblinded randomized trial and a small number of prospective cohort studies were included, the 2017 guidelines make no recommendation regarding which one is better for the diagnosis of CIRCI (3). We acknowledge that adrenocorticotropic hormone (ACTH) stimulation test or a random plasma cortisol is superior to other existing diagnostic tests to establish the diagnosis of CIRCI, but certain supplements might be needed. As we know, critical patients with CIRCI are often complex with various degrees of plasma cortisol hour-to-hour, and then a single random plasma cortisol measurement is imprecise. In addition, the reference range of total or free serum cortisol has not been identified (4). Not all patients suffering from CIRCI display a significant decrease in random plasma cortisol (less than 10 µg/dL). In contrast, a subset of patients has elevated plasma cortisol in the presence of CIRCI (5). The reduced circulating plasma cortisol might partially due to structural damage to the adrenal gland from hemorrhage or infarction (6). Several factors like oxidative stress, cytokines and mediators that suppress cortisol synthesis also lead to decreased plasma cortisol (7). If a random plasma total cortisol <10 µg/dL, a diagnosis of CIRCI might be made just following the guidelines (1,3). But how could we diagnose CIRCI for those who have elevated cortisol or higher than 10 µg/dL? Herein, we provided our own opinions and introduced a potential protocol that might be helpful to improve the diagnosis of CIRCI (Figure 1), and this protocol could be programmed as soon as inadequate GC-mediated anti-inflammatory activity was suspected.
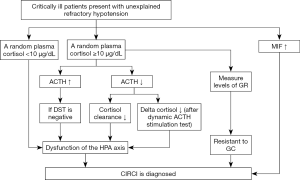
Dexamethasone suppression test (DST)
DST is often used to differentiate diagnosis of hypercortisolism in clinical practice. Dexamethasone can suppress the secretion of hypothalamic and pituitary, inducing the decreased level of cortisol, which can help physicians to judge the function of hypothalamic-pituitary-adrenal (HPA) axis. At the early phase of critical illness or acute stress, HPA axis is activated and stimulates the release of ACTH from the pituitary, which increases the release of cortisol from the adrenal cortex. In such circumstance, patients might have elevated plasma cortisol even in the presence of CIRCI. In addition, for patients with CIRCI, the elevated cortisol concentrations might be partially due to direct production of cortisol from the adrenal glands or ACTH-independent cortisol synthesis (2). Cortes-Puch et al. (8) showed that dexamethasone suppressed cortisol and ACTH levels and restored HPA axis response in survivors with a canine model of severe septic shock due to Staphylococcus aureus pneumonia, while non-survivors who were not influenced by dexamethasone suppression exhibited a distinct HPA axis dysfunction. From these results, we can know that if DST is positive, it suggests that dexamethasone can produce sufficient GC to carry out anti-inflammatory activity, restore the homeostasis, and protect adrenocortical function; if DST is negative, it might indicate the dysfunction of HPA axis and poor prognosis. However, there are no researches on humans with critically illness until now. If this test is proved on human, an increase in the ACTH concentration with a negative response to DST, the dysfunction of HPA axis could be considered.
Cortisol metabolism test
Reducing cortisol breakdown may be another mechanism of CIRCI during critical illness (2). Cortisol is metabolized primarily in the liver and the kidneys, in CIRCI, cortisol clearance is reduced due to the decreased expression and activity of 5-reductases (5β-reductase and 5α-reductase) within the liver and 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) within the kidney (5), the increased half-life and accumulation of cortisol reduce the release of ACTH. Along with the process of stress response, high cortisol levels in turn reduce ACTH concentrations via feedback inhibition. Besides, insufficient blood supply to the anterior pituitary gland, accumulation of nitric oxide or central neuropeptides, and many drugs are associated with decreased ACTH secretion (2). When a patient presents with an elevated cortisol level and a decrease in the ACTH concentration, the cortisol clearance could be estimated by cortisol metabolism test to make sure whether the dysfunction of HPA axis exists. But how to carry out cortisol metabolism test effectively needs to be further investigated. In condition of a decrease in the ACTH concentration with a decreased cortisol clearance by cortisol metabolism test, the dysfunction of HPA axis might be considered.
Dynamic ACTH stimulation test
Evidence has shown that the response to an ACTH stimulation test is poorly reproducible in critically ill patients and adrenal function is dynamic (9). Although ACTH stimulation test may be indicative of adrenal function in certain circumstances, controversy exists with respect to the optimal dose of cosyntropin and end points for assessment (4). A report documented that 20-fold higher incidence of symptomatic adrenal insufficiency in critically ill patients being treated in the ICU for more than 14 days (10). For long-stay patients with decreasing random plasma cortisol (may be still higher than >10 µg/dL), a progressively lowering of the cortisol response to repetitive ACTH stimulation tests might be better than ACTH stimulation test to diagnose CIRCI. In an observational longitudinal retrospective study, de Jong et al. (11) revealed that dynamic changes in delta cortisol using repeated ACTH testing can better reflect adrenal responsiveness and disease severity, independent of baseline cortisol levels and cortisol binding in blood. Dynamic ACTH stimulation test is completed by use of repetitive measurements of plasma cortisol over time together with repetitive ACTH stimulation tests to investigate the changes of cortisol (delta cortisol) (12). Studies have revealed that the cortisol responsiveness to ACTH restores after recovery of illness, while decreasing cortisol responses during dynamic ACTH stimulation test is associated with poor outcomes (11,13). Nevertheless, there are still some problems, for example, how often ACTH stimulation tests should be done and what the optimal dose of cosyntropin is. If these problems are resolved, a decrease in the ACTH concentration with a decreased delta cortisol after dynamic ACTH stimulation tests, the dysfunction of HPA axis could be considered.
Measure levels of GR
Tissue resistance to GC is one of the major pathophysiologic events of CIRCI (2), which refers to decreased GC cellular uptake and/or suppressed expression or function of GR. Critical illness is associated with a decrease in GR-α expression but an increase in GR-β expression, which results in an imbalance between GR-α and GR-β; and these changes likely lead to corticosteroid resistance (14). GR-α is the active receptor, which binds to GC and controls GC-mediated activity, while GR-β has a dominant negative effect on GR-α-induced transcriptional activity (15). Any condition that affects GR-α binding affinity, concentration, transport to the nucleus, interactions with GC-responsive elements, relevant transcription factors (e.g., NF-κB, AP-1) and co-regulators can eventually affect the response of cells to GC (2,16). Meduri et al. (17) demonstrated that deficient GR-α activity was observed despite elevated circulating cortisol and ACTH levels, implicating ACTH stimulation test could not reflect GC resistance. Hence, despite the elevated cortisol levels during critical illness, CIRCI could also occur due to the tissue resistance to GC. Measurement of the functional GR might be a means by which to diagnose CIRCI and assess the usefulness of steroid therapy. Recent study has shown that a more available approach is the real-time measurement of GR nuclear and cytoplasmic levels by flow cytometry using of small quantities of peripheral blood (3–5 mL), which might be of clinical use (15). GR localized to the nucleus has the functional capacity to either activate or repress transcription GR-targeted genes, and it is the localization of GC:GR to the nucleus that determines the therapeutic function. As such, GR localization within the nucleus is a means by which to identify functional GR (15). Unfortunately, the exact levels indicating normal vs. abnormal are still uncertain. If the measurement of GR levels suggests GC tissue resistance, a diagnosis of CIRCI should also be considered.
Biomarker
Biomarker detection is a fast and effective method for diagnosing illness in clinical. Serum macrophage migration inhibitory factor (MIF) is one of the inflammatory mediators, which acts as a physiological counter-regulator of the anti-inflammatory and immunosuppressive effects of GC (18). Multiple clinical studies have shown that MIF is a potential biomarker for different diseases, such as sepsis and septic shock, autoimmune diseases; cancer and metabolic diseases (19). It might also apply to CIRCI. MIF and GC are reciprocally involved in regulating the inflammatory and anti-inflammatory balance (18). At low physiological GC concentrations, MIF synthesis and release are induced, whereas at high GC concentrations its overriding capacity is diminished (20). A recent study demonstrated that patients could be segregated into normal group and adrenal insufficiency group with the threshold serum MIF (19.5 ng/mL) by using receiver operating characteristic (ROC) curve analysis, the maximum sensitivity and specificity for CIRCI were 0.737 and 0.773, respectively (21). Given to the potential role of MIF, it might be a valuable clinical marker of adrenal insufficiency. If the patient has a MIF >19.5 ng/mL, a diagnosis of CIRCI might be established directly. But more randomized control trials are needed to illuminate the relationship between MIF and the HPA axis.
Conclusions
To better understand the diagnosis of CIRCI combined with the guidelines and the review published recently (2,3), we have provided the flow chart though its rationality still needs further confirmation (Figure 1).
Acknowledgements
Funding: This work was supported in part by grants from the National Natural Science Foundation of China (No. 81570017, G Zhang).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 2008;36:1937-49. [Crossref] [PubMed]
- Annane D, Pastores SM, Arlt W, et al. Critical illness-related corticosteroid insufficiency (CIRCI): a narrative review from a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM). Intensive Care Med 2017;43:1781-92. [Crossref] [PubMed]
- Annane D, Pastores SM, Rochwerg B, et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med 2017;43:1751-63. [Crossref] [PubMed]
- Venkatesh B, Cohen J, Cooper M. Ten false beliefs about cortisol in critically ill patients. Intensive Care Med 2015;41:1817-9. [Crossref] [PubMed]
- Boonen E, Vervenne H, Meersseman P, et al. Reduced cortisol metabolism during critical illness. N Engl J Med 2013;368:1477-88. [Crossref] [PubMed]
- Oelkers W. Adrenal insufficiency. N Engl J Med 1996;335:1206-12. [Crossref] [PubMed]
- Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med 2009;360:2328-39. [Crossref] [PubMed]
- Cortés-Puch I, Hicks CW, Sun J, et al. Hypothalamic-pituitary-adrenal axis in lethal canine Staphylococcus aureus pneumonia. Am J Physiol Endocrinol Metab 2014;307:E994-E1008. [Crossref] [PubMed]
- Loisa P, Uusaro A, Ruokonen E. A single adrenocorticotropic hormone stimulation test does not reveal adrenal insufficiency in septic shock. Anesth Analg 2005;101:1792-8. [Crossref] [PubMed]
- Barquist E, Kirton O. Adrenal insufficiency in the surgical intensive care unit patient. J Trauma 1997;42:27-31. [Crossref] [PubMed]
- de Jong MF, Beishuizen A, van Schijndel RJ, et al. Risk factors and outcome of changes in adrenal response to ACTH in the course of critical illness. J Intensive Care Med 2012;27:37-44. [Crossref] [PubMed]
- Goodman S, Sprung CL, Ziegler D, et al. Cortisol changes among patients with septic shock and the relationship to ICU and hospital stay. Intensive Care Med 2005;31:1362-9. [Crossref] [PubMed]
- Ho JT, Al-Musalhi H, Chapman MJ, et al. Septic shock and sepsis: a comparison of total and free plasma cortisol levels. J Clin Endocrinol Metab 2006;91:105-14. [Crossref] [PubMed]
- Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol 2013;132:1033-44. [Crossref] [PubMed]
- Bhatia R, Muraskas J, Janusek LW, et al. Measurement of the glucocorticoid receptor: relevance to the diagnosis of critical illness-related corticosteroid insufficiency in children. J Crit Care 2014;29:691.e1-5. [Crossref] [PubMed]
- Dendoncker K, Libert C. Glucocorticoid resistance as a major drive in sepsis pathology. Cytokine Growth Factor Rev 2017;35:85-96. [Crossref] [PubMed]
- Meduri GU, Muthiah MP, Carratu P, et al. Nuclear factor-kappaB- and glucocorticoid receptor alpha- mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids. Neuroimmunomodulation 2005;12:321-38. [Crossref] [PubMed]
- Calandra T, Bernhagen J, Metz CN, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 1995;377:68-71. [Crossref] [PubMed]
- Grieb G, Merk M, Bernhagen J, et al. Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect 2010;23:257-64. [Crossref] [PubMed]
- Calandra T, Bucala R. Macrophage migration inhibitory factor (MIF): a glucocorticoid counter-regulator within the immune system. Crit Rev Immunol 1997;17:77-88. [Crossref] [PubMed]
- Miyauchi T, Tsuruta R, Fujita M, et al. Serum macrophage migration inhibitory factor reflects adrenal function in the hypothalamo-pituitary-adrenal axis of septic patients: an observational study. BMC Infect Dis 2009;9:209. [Crossref] [PubMed]
Cite this article as: Huang X, Hu W, He X, Zhang G. A potential diagnostic protocol for critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients. J Emerg Crit Care Med 2018;2:86.


