
Probiotics in the critical care unit: fad, fact, or fiction?
Introduction
Probiotics, mostly in their “natural” form, have been used by otherwise healthy people to promote and restore gastrointestinal (GI) health. The term “probiotics” is not new; in fact, probiotics have been around for centuries, mostly consumed in the form of fermented food for the associated lenitive effects. Probiotics are the normally non-pathogenic bacteria, viruses, and fungi that reside in one’s GI tract. These friendly microbes make up the human GI microbiome, or flora, and serve to maintain GI barrier function and integrity, play a role in host nutrient and drug metabolism, immunomodulation, and prevent pathogenic bacteria from colonizing or causing disease. The importance of a healthy microbiome for the overall health of the host is just recently being appreciated within the medical and science communities.
It is widely known that healthy GI flora is important for overall health of the host, and the disruption of this leaves one with increased susceptibility to illness and disease. Here we will provide an overview of the current understanding of the human microbiome, alterations caused to it by critical illness and associated procedures/therapies, and the use of probiotics to restore GI flora for the prevention and amelioration of infection.
Function of the microbiome
Long before existence of microorganisms was known, fermented products were used therapeutically to treat a number of ailments, including fevers, the common cold, and GI distress (nausea and diarrhea). It is now understood that this “normal flora” is part of one’s natural defense against pathogenic invaders. The entire human microbiome consists of viruses, bacteria, fungi, archaea, and single-celled eukaryotes (1). In fact, it is estimated that there are more than 100 trillion bacterial cells comprised of more than 35,000 species that constitute our microbiome (2). Most of these organisms are naturally found in the GI tract, yet others exist as part of the respiratory or genitourinary tract as well as upon our integument (3). Although the healthy gut microbiota consists of varying species and numbers throughout the entire GI tract, nearly 75% of the microbes exist within the large intestine alone (4). When discussing illness or disease related to disruption of the GI flora, it is generally referring to colonic bacteria, and these bacteria usually are what are trying to be restored.
Even ancestral scholars acknowledged the importance of maintaining a healthy GI tract in preventing disease. As early as 400 BC, Hippocrates asserted “death sits in the bowels… a bad digestion is the root of all evil”. The mechanism through which a healthy gut augments one’s immunity is complex and multifaceted, but it can be understood through three barriers of immunity.
The first barrier is the ecological barrier, which is the inhabitant flora of our intestines. The second mechanical barrier resides at the cellular level; it is the intact mucosal epithelia that form a direct, physical barrier against pathogenic organism invasion or translocation of otherwise harmless resident bacteria. The third barrier is an immune barrier, comprised of a slew of host immune cells, including intraepithelial lymphocytes, macrophages, neutrophils, natural killer (NK) cells, aggregated mesenteric lymph nodes (Peyer’s patches) and immunoglobulin A. Disruption of any of these barriers will often have nocuous consequences (5).
As aforementioned, the ecological barrier consists of the trillions of “good” viruses, bacteria, and fungi residing in the human body. Some of the most prevalent and commonly supplemented organisms harbored in the GI tract are of the Bifidobacterium and Lactobacillus species. More specifically, these include strands Bifidobacterium animalis, Bifidobacterium infantis, Bifidobacterium lactis, Lactobacillus casei, Lactobacillus rhamnosus GG, Lactobacillus reuteri, and the yeast Saccharomyces boulardii (6). While each strand of species confers upon the host its own immunologic and nonimmunologic benefits, these organisms collectively modulate a healthy gut and immune system through a variety of similar means. Most importantly, these resident organisms compete against pathogens for binding sites and nutrients utilized as growth substrates (7). Additionally, these friendly organisms produce vitamins (B, K) that can be used for growth of other non-pathogenic organisms, exert anti-inflammatory effects through increasing or decreasing certain cytokine and interleukin activity (8), and stimulating an innate immune response through activation of helper-T cells, macrophages, NK cells, and immunoglobulins (4).
The normal flora also influences the maintenance of mechanical barrier function—the second barrier. When this mechanical barrier of epithelial cells is disrupted, pathogens as well as bacteria which exert no harmful effect inside the GI tract can translocate and cause disease elsewhere. The breakdown of the GI barrier has been linked to several diseases, including: inflammatory bowel disease, chronic kidney disease, necrotizing pancreatitis, celiac disease, food allergy, Clostridium difficile infection (CDI), and sepsis (3,9).
Breakdown and disruption of the GI flora derives from a number of processes, including dehydration and malnutrition, but also may be inadvertently incurred through the administration of antibiotics and other pharmacological therapies (10). The resulting microbial imbalance, or dysbiosis, in turn, affects the third barrier of defense by altering levels of host immune mediators while inducing both chronic inflammation and metabolic dysfunction. What’s more, the composition of the gut microbiome is influenced by various environmental factors, such as lifestyle, diet and hygiene preferences as well as the physiological effects of traumatic injury and critical illness (4,11). Additionally, the procedures and medical therapies that patients are subjected to during hospitalization can further disrupt the GI flora, making a patient even more susceptible to infection. Each of these influences will be discussed in more detail later.
The effect of enteral nutrition on the microbiome
It is understood that a variety of inherent host factors influence the composition and integrity of the microbiome. Perhaps the most important of these is the host’s enteral nutrition, or diet; for, dietary and bacterial metabolites influence immune responses and gut microbiome physiology (12). Therefore, nutritional strategies directed at restoring the natural flora may have particular utility in the critically ill, given that these individuals are most susceptible to alterations of the microbiome. Most of the research has focused on the role fats, carbohydrates, and protein have on gut microbial composition (13). However, greater intake of fiber is thought to strengthen the intestinal barrier, increase peristalsis, and reduce gut inflammation (14).
Fiber, once consumed, is fermented to short chain fatty acids, which include acetate and butyrate. These fatty acids bind G-protein-coupled receptors (GPR43, GPR41), which foster homeostasis and the regulation of inflammatory responses in the gut. More specifically, the G-protein-coupled receptors bound to metabolites augment epithelial integrity and IgA antibody responses (12). Conversely, enteral antibiotic intake may disrupt gut and immune homeostasis by altering the encompassed short-chain fatty acid metabolites, consequently promoting the inflammatory status of the intestinal mucosa (15).
Adapting diets specific to patient needs, including supplementation with probiotics, prebiotics and synbiotics, are possible nutritional strategies for improving gut and microbiome homeostasis. Prebiotics, which are often found as complex carbohydrates in fruits, vegetables and grains, are undigested and unabsorbed until reaching the large intestine where selective fermentation occurs. This promotes the growth and metabolic activity of host flora, which further promotes gut-barrier homeostasis. Probiotics, as previously discussed, may inhibit the growth of enteric pathogens through completive exclusion. They also interact with resident microbiota to modulate host immune function (14).
Critical illness and infection prevention
Increased risk
Patients in critical care units constitute a small percentage of total hospital admissions, yet they account for approximately 25% of all healthcare-associated infections (HAIs) (16). Infection as a complication of critical illness contributes to increased ICU length of stay (LOS), costs associated with admission, resistance to antimicrobials, and morbidity and mortality (17). HAIs are those infections not present and without evidence of incubation at time of admission but develop or become clinically evident after 48 hours of admission. According to the CDC and the results of the 2014 HAI Prevalence Survey, a total of 722,000 HAIs were documented in U.S. acute care hospitals in 2011. What’s more, an astonishing 75,000 patients died as a result of these infections (18). The five most prevalent HAIs were: surgical-site infection (SSI), ventilator-associated pneumonia (VAP), CDI, central-line-associated bloodstream infection (CLABSI), and catheter-associated urinary tract infection (CAUTI) (19). The role that probiotics may play in post-surgery infections, VAP, and CDI will be discussed here. The limited information on CAUTI prevention will also be discussed. The reasons that patients in critical care units incur more infections are many, but the pure nature of critical illness as well as the multitude of treatment modalities necessary for care are major factors.
Disruption of our innate barrier defense mechanisms
The physical barriers afforded by our innate immune response such as skin and the mucosal lining of our respiratory, GI, and urinary tract, can be disrupted either by injury or from procedures common in the critical care unit. Skin barriers are breached by intravenous lines and surgical procedures. Respiratory barriers are compromised by endotracheal intubation. Gastric tubes, inserted nasally or orally, as well as indwelling urinary catheters can disrupt mucosal barriers within our GI/GU systems. Endogenous insult also occurs, perpetrated by certain medications and procedures. All of these serve as means to treat illness, injury, and disease, yet at the same time have infelicitous and puissant consequences.
Common pharmaceutical treatments that alter the microbiota
Antibiotics
It is widely understood and accepted that antibiotics have bactericidal and bacteriostatic effects against both pathogenic and non-pathogenic “good” bacteria. These effects include major changes in the gut microbiota taxonomic diversity which accounts for decreased ability for competitive exclusion. That is, antibiotics destroy multitudes of good bacteria that allow pathogenic bacteria to survive due to less competition for binding sites and growth substrates. However, of principal concern regarding the use of broad-spectrum antibiotics is the opportunity for resistant strains to emerge and be promulgated through horizontal gene transfer among surviving organisms. This accounts for a two-fold insult to human hosts; for, not only are hosts experiencing an alteration of the normal gut microbial diversity, but also pathogenic microbes are being adapted to survive against the current best means for eradication—antimicrobial therapy (4).
It is estimated that more than half of all hospitalized patients received at least one antibiotic during their stay (20). Research demonstrates how GI flora destroyed by just one dose of an antibiotic often takes months to years to recover and host flora may never return to a pre-antibiotic state (21). Further, one-third of antibiotics prescribed in U.S. hospitals involve prescribing problems including prescribing antibiotics for a patient who is not clinically indicated (22). With an understanding of antibiotic-induced dysbiosis, it becomes important for clinicians to focus their efforts on preventing infection and treating HAIs with non-antibiotic strategies whenever possible. Probiotic administration is just one of the suggested strategies for accomplishing this.
H2 receptor blockers/proton pump inhibitors
Prevention of stress-induced ulceration of the GI mucosa is quite common in intensive care units. Usually this is achieved through pharmacological measures such as the administration of H2 receptor blockers and proton-pump inhibitors. Although they offer GI protective effects, the acid secretion suppression and neutralization of GI acidity can be hospitable for a number of pathogenic organisms to flourish, namely Escherichia coli and Clostridium difficile (CD) (23). Other infections associated with an increase in gastric pH and subsequent bacterial overgrowth include pneumonia and bacterial gastroenteritis (24).
Catecholamines
In times of critical illness and stress, the adrenal glands secrete glucocorticoids from the cortex and catecholamines from the medulla in order to activate the sympathetic nervous system (SNS). This “fight or flight” response allows for the body to act upon the perceived threat at hand. In addition to these hormones being secreted endogenously, these substances are administered as exogenous pharmacologic medications commonly meant to support a falling blood pressure along with treatment for a multitude of other conditions. It is known that elevated levels of cortisol and epinephrine contribute to impairment of the immune system, placing the patient at a heightened risk for infection (25).
Opioids
Critical illness is often accompanied by pain, either as a result of an injury or a disease process. Opioid analgesics are used frequently to treat this pain, but also may be used for sedative properties. Regardless of the intended use, opioids possess powerful immunosuppressive properties. Another well-known side effect of opioids is slowing of GI motility. Delayed peristalsis incurred with opioid administration can increase the risk of translocation of bacteria out of the GI system where they can become pathogenic to the host (26). Additionally, treatment with opioids is known to place the patient at an increased risk for CDI due to the associated alterations in the GI microbiome and immune function (27,28).
Probiotics: what are they, how they vary, and how they can restore the microbiome
Probiotics can be simply defined as microbial cells that confer beneficial effects on the health of a human host and are naturally found in many foods (5). Some of the most common probiotic-rich foods include yogurt, cultured vegetables (sauerkraut and kimchi), kefir, and kombucha (see Table 1). However, recent interest has emerged in consuming probiotic supplements in pill, powder, or capsule form to achieve these same benefits. There has been an incredible increase in the number of manufacturers producing and marketing various species and strains of these beneficial microbes to assist in not only digestive health, but also for immune support and as an adjunct to a healthy lifestyle. Under the Dietary Supplement Health and Education Act (DSHEA), a manufacturer of a supplement cannot claim its substance has the capacity to diagnose or cure any particular disease. However, making broad claims such as “improves overall health and well-being” has proven to be quite a draw for the current health-conscious society. Since probiotics mostly are used as a dietary supplement, they do not face stringent regulation by the Food and Drug Administration (FDA).
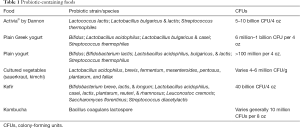
Full table
The fad
The draw of being “healthy” and “boosting immunity” is captivating the interest of both the researcher and the consumer, and manufacturers and marketing companies are capitalizing on this. In fact, the probiotic movement has spread such that you can find probiotics in foods like granola bars, bottled water and juices, and even chocolates. With the growing interest and application of probiotics for specific conditions and overall health claims, these “healthy microbes” will continue to find their way from the shelves to our bellies. Some of the more common and popular brands include: Align®, Culturelle®, Florastor®, Ultimate Flora Extra Care®, Synbiotic 2000 Forte®, Floratrex®, and Raw Probiotics Women® (see Table 2).
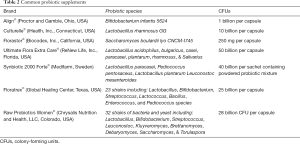
Full table
The facts
Both foods and supplements have been developed and marketed with the intention to enhance wellness in healthy individuals as well as for the dietary management of various diseases. In the U.S., probiotics are regulated as dietary supplements unless a particular product is marketed for having a role in treating or preventing a particular disease. Rather than focusing on quality, safety, and efficacy, regulational oversight focuses on the legitimacy of any claims made by the manufacturer (29). Depending on the intended use, regulatory oversight and requirements for probiotics differ greatly (30). “Nutritional supplements” are considered a food and therefore regulated by the FDA’s Center for Food Safety and Applied Nutrition. However, if the intent is to use a substance for a cure, mitigation, treatment, or prevention of a disease, then it can be classified as a drug by the FDA (31). Manufacturers explicitly state on labeling that the product “is not intended to diagnose, treat, or mitigate disease” in order to be in compliance with the FDA. However, clinicians are often using probiotics for those purposes.
Manufacturing practices, conditions, and ingredients play a major role in the determination of product characteristics and properties. Again, current law may allow for a variety of formulations to be sold under the same brand which may account for a product different from the original. Such regulatory deficits may have dire consequences on consumers as well as for prescribers using these preparations as part of clinical guideline-recommended management of various problems or diseases. A prescriber can be liable for prescribing a formulation of a product not properly tested for safety or efficacy. For these reasons, current regulations are not sufficient to protect consumers or providers. Further regulatory oversight is warranted (29).
As aforementioned, when used for nutritive value, probiotics are considered a supplement and therefore not subject to the scrutiny of the FDA. In light of the recent research examining their use for preventing and treating certain diseases, the FDA responded by defining probiotics as a biotherapeutic product and mandating that any clinical research done with probiotics requires an Investigational New Drug (IND) application when conducting research that is beyond using them as nutritive supplements. This holds probiotics to increased scrutiny and places barriers to advancing research in this area. Manufacturers must provide required information to the FDA. Exclusion criteria set forth by the FDA includes pregnancy, immunosuppression, structural heart disease, or a leaky bowel wall.
The fiction
It is important to dispel a common myth regarding probiotics—all probiotics are not the same. In fact, a particular supplement itself may vary bottle to bottle. Several commercially available products vary between the actual microbial composition (at either the species or genus level) and what is labeled on the container (32). Further, the colony count of species may be far different from labeling, due, in part, to mishandling the product once it leaves the manufacturing facility. Accounting for this variability is the lack of regulation and oversight over the probiotic market. Probiotics are live organisms and are extremely sensitive to their environment. The manufacturing process and handling of the products themselves are not always the same. In addition, many of the properties of probiotics are not only species but strain specific (33). This means that safety and efficacy of findings should not be generalized to similar products (29).
Use of probiotics/evidence-based use of probiotics
VAP
VAP, a type of hospital acquired pneumonia, is the second most common nosocomial infection in the U.S. and the most frequent HAI in intensive care units (34). In fact, it is estimated that as many as 30% of mechanically ventilated patients develop VAP (35). Similar to other HAIs, VAP is associated with poor clinical outcomes and high financial burden, with estimates close to an additional $40,000 of hospital costs per patient (34). VAP can be diagnosed in any critical care patient who has been mechanically ventilated for at least 48 hours and demonstrates clinical symptoms of pneumonia along with displaying relevant radiographic criteria (36). VAP has long been an outcome indicator of quality of care and infection prevention strategies among critical care units. More recently, the concept of infection-related ventilator complications (IVAC) and ventilator associated complications (VAC) have been proposed by the CDC to expand upon VAP as more objective measures of quality of care, since the diagnostic criteria of VAP may be interpreted differently by clinicians (37). A further discussion on the nosology and etiology of VAP, IVAC, and VAC are beyond the scope of this discussion.
Bundles of care specific to ventilated patients have been proposed and implemented across the world as effective healthcare-associated pneumonia prevention strategies (37). It is predicted that incidence of VAP can be decreased by 50–60% if evidence-based care bundles are instituted and properly followed by all medical staff a part of the care of mechanically ventilated patients (38). Essential components of these bundles, as explicated by the Institute for Healthcare Improvement, include: daily interruption of sedation with wakening and weaning trials, elevation of the head of the bed to at least 45 degrees at all times, deep vein thrombosis prevention with pharmaceuticals and sequential compression devices, pharmacologic prevention of intestinal bleeding and gastric ulcers, and frequent oral care, perhaps with chlorhexidine (39). In light of current research regarding the efficacy of probiotics on decreasing incidence of VAP, it may be worth considering probiotic supplements as part of ventilator care bundles.
With an understanding of how probiotics positively influence host gut-barrier health, it should come as no surprise that there is a growing body of evidence demonstrating the positive effects probiotics have on incidence of hospital-acquired infections, including VAP. Probiotic therapy may prevent and treat VAP by restoring non-pathogenic bacteria that compete with pathogens for binding sites and growth substrates, modulating host immune response, and augmenting gut mucosal barrier function. One particular species of probiotics substantiated by the literature regarding safety and efficacy is Lactobacillus rhamnosus GG (LGG). In a study by Morrow et al., administration of LGG was associated with a significant reduction in the incidence of VAP with microbiological confirmation on invasive lower respiratory tract samples (40). However, when examining the total body of evidence pertaining to the therapeutic use and safety of probiotics, there remains inconsistency of results (34). Further research is warranted to better understand what exact species and dosing as well as what time is ideal for introduction to the host to prevent and combat VAP.
CDI
CD and CDI is a frequent cause of hospital acquired infection and significantly increases a patient’s morbidity and mortality. In 2008, 66 out of 100,000 patients were infected with CD; a rate which doubled from that just eight years prior. Risk factors for CDI are advanced age (age greater than age 65), prolonged hospital stay, female sex, immunocompromised, and recent antibiotic administration, among others (41). In fact, just one dose of an antibiotic can severely alter a host’s microbiome to the point where opportunistic pathogens like CD can proliferate and cause illness for an extended period of time (42). Current recommended treatment approaches include metronidazole for mild to moderate cases of CDI and vancomycin for severe cases. These antibiotics often result in recurrent CDI, however, due to their broad-spectrum coverage that destroys not only CD but also host microbiota. Recent studies aspire to find a new, narrow-spectrum antibiotic, such as thuricin CD, with specific anti-CD coverage to reduce the collateral destruction on host microbiota (42).
In addition to the effect a more narrow-spectrum antibiotic may have on mitigating the destruction of host flora during treatment of CD, concurrent supplement with probiotics may prove beneficial. A recent meta-analysis by Johnson et al. found moderate-quality evidence suggesting that supplemental therapy with probiotics resulted in a significant reduction in incidence of CD-associated diarrhea without any association of increased adverse events. In fact, when examining 20 trials that included over 3,800 patients, Johnson et al. saw a 66% reduction of CD-associated diarrhea when patients were supplemented with species of Bifidobacterium, Lactobacillus, Saccharomyces and/or Streptococcus in patients receiving antibiotics. Trials that used multiple species showed greater effects than those using a single species. Their findings offer reason to encourage the use of probiotics in patients receiving antibiotics who are at risk for CDI and CD-associated diarrhea (43).
Furthermore, Lau and Chamberlain also conducted a meta-analysis to ascertain the efficacy of probiotics on reducing the incidence of CDI. They examined 26 randomized controlled trials that included a total of 7,957 patients. Lau and Chamberlain found that probiotics had an effect of 60.5% reduction on the incidence of CD-associated diarrhea. Specifically, treatment with Lactobacillus, Saccharomyces, or a mixture of probiotic species reduced CD-associated diarrhea by 63.7%, 58.5%, and 58.2%, respectively. Again, the variation and heterogeneity of trials accounts for a major limitation of findings; however, probiotics should still be considered a valuable adjunct in the therapeutic regimen of patients receiving antibiotics unless otherwise contraindicated (44). Finally, Goldenberg and colleagues conducted a Cochrane review that found probiotics decreased the risk of CD-associated diarrhea by 60% in patients who were not immunocompromised or in a severely debilitated state. Based on data from this meta-analysis of 31 randomized controlled trials including over 8,670 patients, researchers concluded that moderate certainty evidence suggests that concurrent probiotic use along with antibiotics is a safe and effective strategy for preventing CD-associated diarrhea (45).
CAUTI
A urinary tract infection (UTI) occurs when the urethra is colonized with uropathogens or fecal flora. These organisms include E. coli (50%), Proteus (15%), Enterobacter (15%), and Klebsiella (15%), among others with less significant rates. Uropathogens can further spread down the urethra to the bladder and possibly the kidneys via the ureters. Such UTIs account for many cases of cystitis and pyelonephritis (46). What’s more, significant comorbidities resulting from UTIs include urethral stricture, abscess or fistula formation, bacteremia, and sepsis. An astonishing 25% of all sepsis cases emanate from UTIs (47).
Among all cases of healthcare-associated UTI, approximately 75% are associated with a urinary catheter. It is estimated that between 15–25% of hospitalized patients receive urinary catheters at some point in their stay (48). Indwelling urinary catheters are often necessary for critically ill patients in order to monitor intake and output and to alleviate urinary outflow obstruction, for example, but they are a major source of preventable infection. Improper handling and poor hygiene can result in contamination of the catheter which allows for pathogenic organisms to invade the urinary tract. Additionally, urinary catheter drainage bags serve as potent bacterial reservoirs.
The risk of contracting a CAUTI increases with prolonged use of the catheter. Therefore, urinary catheters should be removed as soon as clinically warranted. Unfortunately, the research regarding the efficacy of probiotics on the prevention of CAUTI is limited. At this time, there is not enough evidence to assert that probiotics may be an effective choice for CAUTI prophylaxis. However, in light of recent research regarding the efficacy of probiotics for preventing UTIs in general, studies with the intent to ascertain the efficacy of probiotics for prevention of CAUTI specifically is warranted.
Recently, researchers sought to review the safety and efficacy of probiotics in the prevention of UTI, given that probiotics have other potential uses as prophylactic therapies. Schwenger et al. reviewed the effect probiotics had on morbidity and mortality compared to placebo or no therapy in patients susceptible to UTI. They included nine studies that involved over 730 patients. The focus of these studies was to quantify differences in incidence of recurrent UTI. Researchers found that no significant benefit with probiotics therapy compared with placebo or no treatment; however, a benefit cannot be ruled out for several crucial reasons. These include a data set that was too limited and use of small studies with poor methodological reporting (47).
SSI
Roughly 1.5 million of the 80 million surgeries performed in the U.S. each year is complicated by a SSI. Post-SSIs are the most common of all HAIs, comprising approximately 30% of the total number of these infections. SSIs can add 7 to 11 additional postoperative days to a patient’s stay and increase mortality by up to 11 times. Among perioperative patients, 77% of deaths are directly attributed to SSIs (49). Septic morbidity and mortality associated with surgical and medical treatments is high and rising all over the world, with estimates around 200,000 annual deaths in the U.S. attributed to sepsis (50). Researchers and clinicians are continuously exploring ways to reduce the incidence of infective complications and other surgical adverse events in perioperative patients. Evidence suggests that surgical trauma disrupts the gut microbiome and allows for translocation of normal gut flora. The gut itself is closely linked to the initiation of systemic inflammatory processes that are, in part, responsible for the development of sepsis following surgery.
Recently, perioperative nutrition modulation of gut flora is increasingly being used as an outcome improvement strategy. Probiotic, prebiotic, and synbiotic therapies are being substantiated by the literature as effective means to mitigate the disruptive effect surgical trauma has on gut function. For reference, a prebiotic is a food substance that positively influences growth and survival of host microbiota and a synbiotic is a product that contains both prebiotics and probiotics (51).
A meta-analysis performed by Kinross et al. explored the effect probiotic and synbiotic therapy had on postoperative sepsis rates (52). Over 960 elective surgery patients from 13 randomized controlled trials were sampled with the primary outcome measure being postoperative sepsis rate. Of the 962 patients, 304 received synbiotics and 182 received probiotics either postoperatively or pre- and postoperatively. Probiotics used were of the Enterococcus, Lactobacillus, Lactococcus, Leuconostoc, and Bifidobacterium species. Researchers found that the rate of postoperative sepsis was reduced in both the probiotic and synbiotic groups compared to the control group.
Prevention above all else
Although decreasing costs associated with the development of infections is important, the main reason to prevent HAIs is to improve outcomes and save lives. Deaths attributed to critical illness/sepsis have been increasing more quickly than any other cause of mortality in this population, making infection prevention strategies even more critical (34).
Safety and risks associated with probiotics
The rapidly expanding consumer probiotic market is reaching into the acute care setting. While research with probiotics for the prevention and treatment of certain ailments in otherwise healthy people is not new, the theoretical benefit of restoring a healthy microbiome for a person that is acutely ill and at increased risk for infection is being explored with great interest. Their use in the acute care setting has been quite promising, but the use of probiotics in the critical care setting has caused significant controversy. Research with probiotics demonstrates their efficacy in preventing and treating various conditions, particularly those involving the GI tract, but can they be harmful? There are certain patient populations in whom risks versus benefits must be carefully considered. Although the overwhelming evidence supports that probiotics are safe, there are case reports of risks associated with probiotic use as well as some theoretical risks that have been posed in certain patient populations.
The most widely cited safety concern surrounding probiotics is the possibility for bacteremia and fungemia associated with their use in certain high risk groups. Adverse effects of probiotics are not widely reported in studies, yet case reports of infection possibly related to concomitant probiotic use have been reported. Most of these infections occurred in “vulnerable” groups (53). Even though their overall safety has been confirmed in literature reviews, caution still needs to be taken in certain situations.
A systematic review published in 2014 concluded that probiotics are safe, yet caution should be taken when using probiotics in populations such as those that are critically ill in the recent postoperative period and immunocompromised (54). Case reports of infection also have been reported in patients with short bowel syndrome, central venous catheters, and patients with cardiac valve disease or mechanical heart valves (53,55,56). The risk of infection may also be related to improper handling of the probiotics themselves.
Systemic infections have been cited; cases of Lactobacillus bacteremia and fungemia associated with the administration of Lactobacillus probiotics and Saccharomyces species in patients who also had central lines have been reported (30,55,57,58). These preparation (usually capsules or sachets) often need opened for administration through a feeding tube which is a practice that can potentially spread the microbes into the air and cause them to contaminate the hands of healthcare workers (59). Improper hand washing, then, can cause a translocation to a central line catheter where the microbes have direct entry into systemic circulation. Although the manufacturers of some of these products list on the label that they are not to be administered to patients with central lines, this practice still does occur. Precautions must be taken to avoid the accidental contamination of the central line of the patient receiving the probiotic or those with central venous catheters in the close proximity to the patient receiving the probiotic (60).
Other safety concerns have been cited by researchers and healthcare practitioners, including the possibility of gene transfer from the microbe itself, toxins being produced, and effects on a person’s immunological system. It is theoretically possible for certain microbes to transfer gene resistance to the host. Certain Lactic acid bacteria have genes resistant to common antibiotics such as macrolides and chloramphenicol (55). Although this does remain a possibility, no literature to date supports this theoretical risk to humans.
Commercially available probiotics may contain a single or multiple strain of a particular microbial species. This could either be multiple strains of the same species, or strains from more than one genus (29). Different products contain varying amounts of bacteria or fungi, so safety related to the quantity of living microbes being ingested is a concern. Additionally, with each species or strain added to a preparation, concern regarding the adverse effect profile, or safety, of each is necessary.
It is known that probiotics affect both our innate and adaptive immune systems, so concern has been raised over the possibility of over-stimulating immune function in certain individuals. Theoretically, this could lead to an “awakening” of an autoimmune disease in the host. However, this too has not been evident in the literature thus far (8).
There is a scarcity of studies that specifically examine the safety profile of probiotics when administered to the critically ill. Considering the long history of safe use of probiotics coupled with the actual and potential risks associated with improper administration or certain vulnerable groups, it is best for clinicians and researchers to take precautions. This includes conducting a careful risk/benefit assessment for certain patient groups. When using probiotics, active surveillance for cases of infection associated with probiotic use along with laboratory confirmation of causative organisms and vigilant reporting of same is important for establishing a safety profile for their use in the critically ill population.
Conclusions
Despite lack of clear, scientific evidence on efficacy, the appeal for consumption and sale of probiotic supplements and probiotic foods continues to grow. Beyond the role they play in promoting GI health, probiotics have been studied and shown to facilitate restoration of the microbiome. Despite lack of homogeneity and the number of high quality studies, published research shows much promise for the use of probiotics to restore altered microbiota and therefore confer substantial benefits to the critically ill; namely the prevention and, in some cases, amelioration of infection. Therefore, an ethical conundrum exists for healthcare providers and a risk-benefit ratio must be explored. Does the administration of probiotics to critically ill patients, despite lack of clear clinical guidelines, offer significant benefit? Certainly, a precautionary approach is warranted (61). It is important for clinicians to distinguish between the reality of what is presented in the marketing of probiotics, what is known from research, and purely theoretical benefits and potential harms associated with their use. In essence, current evidence supports a role for probiotics in the critical care setting. However, results, in their current form, must be interpreted cautiously in order to ascertain what this legitimate role may be and which species and strains will provide the most benefit.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sekirov I, Finlay BB. Human and microbe: United we stand. Nat Med 2006;12:736-7. [Crossref] [PubMed]
- Frank DN, Amand ALS, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 2007;104:13780-5. [Crossref] [PubMed]
- Reid G, Younes JA, Van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 2011;9:27-38. [Crossref] [PubMed]
- Jandhyala SM, Talukdar R, Subramanyam C, et al. Role of the normal gut microbiota. World J Gastroenterol 2015;21:8787. [Crossref] [PubMed]
- Fioramonti J, Theodorou V, Bueno L. Probiotics: What are they? What are their effects on gut physiology? Best Pract Res Clin Gastroenterol 2003;17:711-24. [Crossref] [PubMed]
- Surawicz CM. Probiotics, antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in humans. Best Pract Res Clin Gastroenterol 2003;17:775-83. [Crossref] [PubMed]
- Morrow LE, Gogineni V, Malesker MA. Probiotics in the intensive care unit. Nutr Clin Pract 2012;27:235-41. [Crossref] [PubMed]
- Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: A prospective randomized pilot study. Crit Care 2011;15:R290. [Crossref] [PubMed]
- Morrow LE, Wischmeyer P. Blurred lines: Dysbiosis and probiotics in the ICU. Chest 2017;151:492-9. [Crossref] [PubMed]
- Wolff NS, Hugenholtz F, Wiersinga WJ. The emerging role of the microbiota in the ICU. Crit Care 2018;22:78. [Crossref] [PubMed]
- Dickson RP. The microbiome and critical illness. Lancet Respir Med 2016;4:59-72. [Crossref] [PubMed]
- McKenzie C, Tan J, Macia L, et al. The nutrition‐gut microbiome‐physiology axis and allergic diseases. Immunol Rev 2017;278:277-95. [Crossref] [PubMed]
- Biesalski HK. Nutrition meets the microbiome: Micronutrients and the microbiota. Ann N Y Acad Sci 2016;1372:53-64. [Crossref] [PubMed]
- Salazar N, Valdés-Varela L, González S, et al. Nutrition and the gut microbiome in the elderly. Gut Microbes 2017;8:82-97. [Crossref] [PubMed]
- Agostoni C, Kim KS. Nutrition and the microbiome. Pediatr Res 2015;77:113-4. [Crossref] [PubMed]
- Fawzy M, Genena D, Sewify K. Should probiotics be routinely used in critically ill patients? BAOJ Nutrition 2017;3:043.
- Crooks NH, Snaith C, Webster D, et al. Clinical review: Probiotics in critical care. Crit Care 2012;16:237. [Crossref]
- CDC. HAI Data 2018. Available online: https://www.cdc.gov/hai/surveillance/index.html
- Zimlichman E, Henderson D, Tamir O, et al. Health care–associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 2013;173:2039-46. [Crossref] [PubMed]
- Baggs J, Fridkin SK, Pollack LA, et al. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 2016;176:1639-48. [Crossref] [PubMed]
- Stavrou G, Kotzampassi K. Gut microbiome, surgical complications and probiotics. Ann Gastroenterol 2017;30:45. [PubMed]
- CDC. National action plan for combating antibiotic resistant bacteria 2015. Available online: https://www.cdc.gov/drugresistance/pdf/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf
- Bavishi C, Dupont H. Systematic review: The use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther 2011;34:1269-81. [Crossref] [PubMed]
- Kanno T, Matsuki T, Oka M, et al. Gastric acid reduction leads to an alteration in lower intestinal microflora. Biochem Biophys Res Commun 2009;381:666-70. [Crossref] [PubMed]
- Oberbeck R. Catecholamines: Physiological immunomodulators during health and illness. Curr Med Chem 2006;13:1979-89. [Crossref] [PubMed]
- Balzan S, de Almeida Quadros C, De Cleva R, et al. Bacterial translocation: Overview of mechanisms and clinical impact. J Gastroenterol Hepatol 2007;22:464-71. [Crossref] [PubMed]
- Wang F, Meng J, Zhang L, et al. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep 2018;8:3596. [Crossref] [PubMed]
- Mora AL, Salazar M, Pablo-Caeiro J, et al. Moderate to high use of opioid analgesics are associated with an increased risk of Clostridium difficile infection. Am J Med Sci 2012;343:277-80. [Crossref] [PubMed]
- de Simone C. The unregulated probiotic market. Clin Gastroenterol Hepatol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Venugopalan V, Shriner KA, Wong-Beringer A. Regulatory oversight and safety of probiotic use. Emerg Infect Dis 2010;16:1661-5. [Crossref] [PubMed]
- Dietary supplements: Questions and answers.: U.S. Food and Drug Administration; 2015. Available from: https://www.fda.gov/drugs/resourcesforyou/consumers/../ucm100102.htm
- Huys G, Vancanneyt M, D'Haene K, et al. Accuracy of species identity of commercial bacterial cultures intended for probiotic or nutritional use. Res Microbiol 2006;157:803-10. [Crossref] [PubMed]
- McFarland LV, Evans CT, Goldstein EJ. Strain-specificity and disease-specificity of probiotic efficacy: a Systematic review and meta-analysis. Front Med (Lausanne) 2018;5:124. [Crossref] [PubMed]
- Manzanares W, Lemieux M, Langlois PL, et al. Probiotic and synbiotic therapy in critical illness: A systematic review and meta-analysis. Crit Care 2016;19:262. [Crossref] [PubMed]
- Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia. Am J Respir Crit Care Med 2010;182:1058-64. [Crossref] [PubMed]
- Neuville M, Mourvillier B, Bouadma L, et al. Bundle of care decreased ventilator-associated events—implications for ventilator-associated pneumonia prevention. J Thorac Dis 2017;9:430. [Crossref] [PubMed]
- Timsit JF, Esaied W, Neuville M, et al. Update on ventilator-associated pneumonia. F1000Res 2017;6:2061. [Crossref] [PubMed]
- Bouadma L, Wolff M, Lucet JC. Ventilator-associated pneumonia and its prevention. Curr Opin Infect Dis 2012;25:395-404. [Crossref] [PubMed]
- Niederman MS. New strategies to prevent ventilator-associated pneumonia: What to do for your patients. Curr Treat Options Infect Dis 2016;8:1-15. [Crossref]
- Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: A blinded, randomized, controlled trial. Am J Respir Crit Care Med 2010;182:1058-64. [Crossref] [PubMed]
- Bommiasamy AK, Connelly C, Moren A, et al. Institutional review of the implementation and use of a Clostridium difficile infection bundle and probiotics in adult trauma patients. Am J Surg 2018;215:825-30. [Crossref] [PubMed]
- Rea MC, Dobson A, O'Sullivan O, et al. Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc Natl Acad Sci U S A 2011;108 Suppl 1:4639-44. [Crossref] [PubMed]
- Johnson S, Maziade PJ, McFarland LV, et al. Is primary prevention of Clostridium difficile infection possible with specific probiotics? Int J Infect Dis 2012;16:e786-92. [Crossref] [PubMed]
- Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile-associated diarrhea: A systematic review and meta-analysis. Int J Gen Med 2016;9:27. [PubMed]
- Goldenberg JZ, Ma SS, Saxton JD, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2013;5:CD006095. [PubMed]
- Porat A, Kesler S. Urosepsis. Updated 2018 Oct 13. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2018 January.
- Schwenger EM, Tejani AM, Loewen PS. Probiotics for preventing urinary tract infections in adults and children. Cochrane Database Syst Rev 2015:CD008772. [PubMed]
- CDC. Prevention of CAUTI; Catheter-associated Urinary Tract Infections (CAUTI) 2015. updated July 19, 2017. Available online: https://www.cdc.gov/hai/ca_uti/uti.html
- Awad SS. Adherence to surgical care improvement project measures and post-operative surgical site infections. Surg Infect (Larchmt) 2012;13:234-7. [Crossref] [PubMed]
- Bengmark S. Pro-and synbiotics to prevent sepsis in major surgery and severe emergencies. Nutrients 2012;4:91-111. [Crossref] [PubMed]
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 1995;125:1401-12. [PubMed]
- Kinross JM, Markar S, Karthikesalingam A, et al. A meta-analysis of probiotic and synbiotic use in elective surgery: Does nutrition modulation of the gut microbiome improve clinical outcome? JPEN J Parenter Enteral Nutr 2013;37:243-53. [Crossref] [PubMed]
- Hempel S, Newberry S, Ruelaz A, et al. Safety of probiotics to reduce risk and prevent or treat disease. Evidence Reports/Technology Assessments, No. 200, 2011.
- Didari T, Solki S, Mozaffari S, et al. A systematic review of the safety of probiotics. Expert Opin Drug Saf 2014;13:227-39. [Crossref] [PubMed]
- Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis 2015;60:S129-34. [Crossref] [PubMed]
- Williams NT. Probiotics. Am J Health Syst Pharm 2010;67:449-58. [Crossref] [PubMed]
- Enache-Angoulvant A, Hennequin C. Invasive Saccharomyces infection: a comprehensive review. Clin Infect Dis 2005;41:1559-68. [Crossref] [PubMed]
- Salminen SJ, Gueimonde M, Isolauri E. Probiotics that modify disease risk. J Nutr 2005;135:1294-8. [Crossref] [PubMed]
- Hennequin C, Kauffmann-Lacroix C, Jobert A, et al. Possible role of catheters in Saccharomyces boulardii fungemia. Eur J Clin Microbiol Infect Dis 2000;19:16-20. [Crossref] [PubMed]
- Skljarevski S, Barner A, Bruno-Murtha LA. Preventing avoidable central line-associated bloodstream infections: Implications for probiotic administration and surveillance. Am J Infect Control 2016;44:1427-8. [Crossref] [PubMed]
- Vitko HA, Sekula LK, Schreiber MA. Probiotics for trauma patients: Should we be taking a precautionary approach? J Trauma Nurs 2017;24:46-52. [Crossref] [PubMed]
Cite this article as: Vitko HA, Troxell JJ. Probiotics in the critical care unit: fad, fact, or fiction? J Emerg Crit Care Med 2018;2:95.


