Effect of obesity on propofol dosing requirements in mechanically ventilated patients in a medical intensive care unit
Introduction
The obese population has nearly doubled in the past 3 decades worldwide. Nearly one third of patients admitted to the intensive care unit (ICU) are obese with 7% of these patients meet criteria for morbid obesity (1,2). Critically ill hospitalized patients display large variations in pharmacokinetics for many medications due to altered absorption, distribution, metabolism, or elimination; obesity often compounds this phenomenon. Obese patients have an increase in both lean mass and adipose tissue, additionally, average cardiac output is higher due in part to increased intravascular volume (2,3). Excess adipose tissue results in an increase in volume of distribution (Vd) of lipophilic medications, confounding dosing of these drugs (2). Current sedation and analgesia guidelines in critically ill patients recommend several lipophilic drugs as first-line agents for sedation, though little is known about initial dose and titration for these agents in the obese patient (4).
Propofol is a highly lipophilic, short acting, general anesthetic commonly used in intubated, mechanically ventilated patients. A three-compartment model of distribution most often characterizes the pharmacokinetic profile of propofol. The apparent Vd increases with the length of the continuous infusion, due to drug accumulation in deep tissue compartments (5). This effect is exaggerated in obesity, in theory leading to increased accumulation and saturation of deep tissues. This leads to longer time to elimination after the infusion is terminated as drug distributes back into the central circulation (2,6,7). These alterations would be predicted to decrease time to achieve adequate sedation (when dosed by actual body weight) and prolong the time to arousal allowing extubation. If daily “wake-ups” are utilized, the latter issue may be mitigated. Several past studies have described propofol dosing in obese patients undergoing elective surgeries. In these limited reports, investigators concluded that when propofol was dosed based on total body weight, there was a shorter time to loss of consciousness, but otherwise no large differences between non-obese and obese patients were observed (3,8,9).
The objective of this study was to investigate the hypothesis that altered dose-effect of propofol infusions in intubated, mechanically ventilated obese patients significantly alters clinical sedation parameters, dosing requirements, and propensity towards adverse events.
Methods
This single center, retrospective chart review was conducted at NYU Langone Medical Center (NYULMC), a 726-bed tertiary care academic medical center. The NYULMC Institutional Review Board granted exemption from the need for informed consent. Electronic charts of patients admitted to the MICU who were prescribed propofol from January 2013 to March 2015 were reviewed sequentially. Eligible patients were ≥18 years of age, intubated and mechanically ventilated for at least 24 hours in the MICU, received a continuous propofol infusion dosed per kilogram of actual body weight targeted to achieve and maintain light to moderate sedation defined by a goal RASS score of 0 to −3. Patients were excluded if they had received an opioid infusion for greater than 24 hours prior to propofol initiation (PRN doses were permitted), received benzodiazepines or dexmedetomidine before or during propofol use, received concomitant neuromuscular blockade, were pregnant, had known chronic benzodiazepine and/or illicit drug use prior to admission, or had a primary acute neurologic impairment altering assessment of RASS scores (stroke, traumatic brain injury, anoxic brain injury, etc.).
Data was extracted via review of electronic health records. Collected variables included age, gender, ideal body weight, actual body weight, body mass index (BMI), MICU diagnosis, and pertinent medication history. Vital signs preceding and during propofol infusion and pertinent laboratory values such as triglycerides, transaminases, creatinine kinase, lipase, daily RASS scores and corresponding infusion rates of propofol were recorded; use of sedation/analgesia and vasoactive medications were also evaluated.
Our primary outcome was the initial rate of propofol infusion required to achieve goal RASS. Secondary endpoints were median infusion rate of propofol per day, median infusion rate of fentanyl per day, time from discontinuation of propofol drip to extubation, total duration of sedation and mechanical ventilation, occurrence of ventilator-associated pneumonia and re-intubations, ICU and hospital length of stay, and mortality. Adverse events examined included the incidence of hypotension and bradycardia within 24 hours of propofol initiation, incidence of ≥10% decrease in ejection fraction during infusion, increased triglycerides, increases in creatinine kinase, and evidence of propofol-related infusion syndrome (PRIS).
Endpoints were compared in two groups: non-obese (BMI <30 kg/m2) and obese patients (BMI ≥30 kg/m2). A subgroup of morbidly obese patients with BMI ≥40 kg/m2 was taken from the obesity group and endpoints were compared to the control group. Patients were considered to meet goal RASS if their score was ±1 from documented goal RASS. Bradycardia was defined as a heart rate of ≤50 beats per minute. Hypotension was defined by an increase in vasopressor requirements and/or a ≥30% decrease in systolic blood pressure within 24 hours of starting the propofol infusion.
Data was analyzed using SPSS statistical software package version 21, categorical variables by the Pearson Chi-squared test and Phi and Cramer’s test, and continuous data by the Mann Whitney U test. P values of <0.05 were considered statistically significant. A power calculation was not assessed for sample size secondary to lack of previous data to estimate the effect of obesity on propofol dosing and that this was a descriptive, observational study aimed to prompt prospective studies of a larger scale.
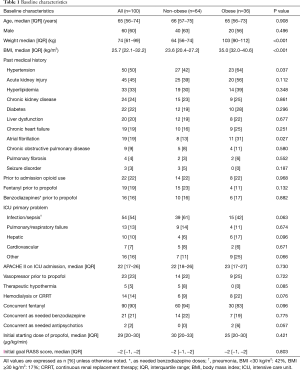
Results
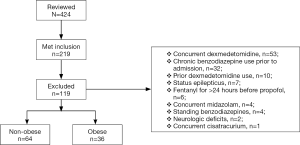
A total of 424 charts were reviewed and 219 met inclusion criteria; 119 were excluded, predominantly due to antecedent or concurrent medication use, most commonly, the concurrent use of dexmedetomidine (n=53), chronic benzodiazepine use prior to admission (n=32), and prior dexmedetomidine use (n=10). Therefore, 100 patients were included in the study: obese (n=36) and non-obese (n=64) (see Figure 1). The subgroup of morbidly obese patients included 11 patients of the overall obese patient group. The majority of subjects were male with a median age of 65 years. Average BMI was 35.0 vs. 23.6 kg/m2 (P<0.001), and obese patients had a higher baseline incidence of hypertension and atrial fibrillation. Sepsis was the most common ICU admission diagnosis (54%) in both groups. Median APACHE II score was 22 and 23 in the non-obese group vs. obese group, respectively (P=0.730). In the non-obese group 22% of patients were on vasopressors prior to propofol administration vs. 25% in the obese group (P=0.722). The median initial starting infusion rate of propofol for both groups was 29 µg/kg/min and the initial goal RASS scores for both groups was −2 (Table 1).
Full table
For the primary outcome measure, obese patients required lower initial doses of propofol infusion in order to achieve goal RASS (20 vs. 30 µg/kg/min, P=0.037). The median time to goal RASS was 6 hours in the obese group and 9 hours in the non-obese (P=0.193). Approximately 17% of patients in both groups were over sedated and did not meet goal RASS (Table 2).
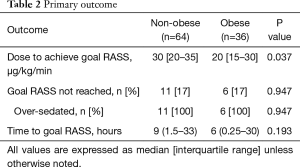
Full table
Median infusion rate per day was separated by individual goal RASS scores of 0, −1, −2, and −3 to better observe changes in infusion rates between groups in context to specific RASS scores. Median infusion rate for RASS of 0 was significantly higher in the non-obese group vs. the obese group on day 3 (P=0.012). Infusion rates for RASS of −2 were significantly different on day 2 (P<0.001) and day 3 (P=0.025). Median infusion rates to achieve RASS of −3 were significantly lower in the obese group on day 1 (P=0.043), day 3 (P=0.006), day 4 (P=0.009), and day 5 (P<0.001) (Table 3).
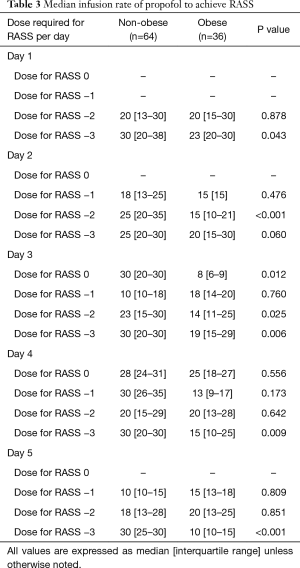
Full table
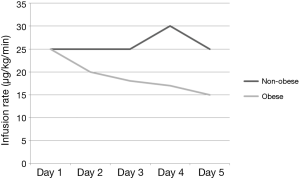
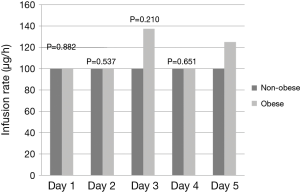
Obese patients required significantly lower median infusion rates of propofol per day to maintain goal RASS (Figure 2). Obese patients were maintained on propofol for a median of 58 vs. 49 hours in the non-obese group (P=0.920). After discontinuation of propofol, 50% of the patients in both groups were continued on other sedatives before extubation yielding a total sedation time for obese patients of 92 vs. 75 hours with the non-obese group (P=0.641). Time from discontinuation of propofol to extubation was 7 hours in the obese group vs. 2 hours in the non-obese (P=0.236). The median duration of mechanical ventilation was 99 in the obese group vs. 75 in the non-obese (P=0.799). Re-intubations did not differ between the groups with 8% in the obese group and 13% in the non-obese (P=0.523). Ventilated associated-pneumonia occurred in 14% of the obese group and 11% in the non-obese group (P=0.663) (Table 4). Fentanyl infusion rates per day did not differ significantly between the groups and stayed relatively stable (Figure 3). ICU and hospital length of stay and ICU mortality were not significantly different between the groups. However, hospital mortality was higher in the obese group vs. the non-obese group (56% vs. 31%, P=0.017) (Table 4).
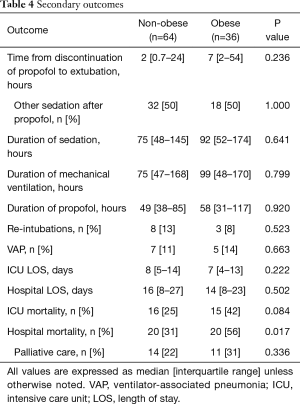
Full table
Adverse events within 24 hours of propofol administration, predominantly hypotension, did not differ between the two groups and none of the patients experienced bradycardia during propofol administration. Echocardiograms were available for eight patients in the obese group and 20 patients in the non-obese group. Fall in ejection fraction was common but not different between groups. Evaluated lab values such as triglycerides, creatinine kinase and clinical development of PRIS were rare and similar in the two groups (Table 5).
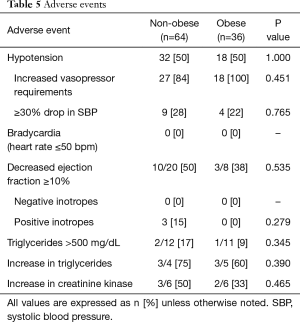
Full table
In the subgroup of morbidly obese patients the median infusion rates per day of propofol on day 4 was significantly less in the morbidly obese (15 vs. 30 µg/kg/min) in the non-obese patients (P=0.039). No other parameter was statistically different in the 2 subgroups of obese patients (Table 6).
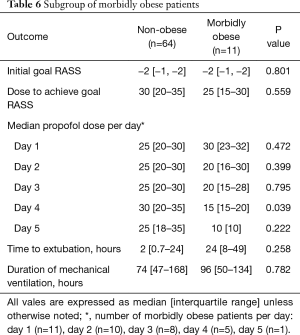
Full table
Discussion
Propofol is a short acting, lipophilic sedative that induces central nervous system (CNS) depression by agonism of GABAA receptors, and antagonism of N-methyl-D-aspartate (NMDA), nicotinic, and M1 muscarinic receptors (5). The current critical care guidelines on sedation and analgesia recommend propofol as a first-line agent for sedation in the ICU due to rapid onset and offset effects (4). The lipophilicity of propofol allows rapid penetration into the CNS and rapid redistribution out of the CNS once an infusion is discontinued.
The pharmacokinetics of a propofol infusion is most often characterized by a three-compartment model. As the duration of the infusion increases, the volume of distribution predictably increases. Propofol promptly distributes into the central compartment, which contains the brain and the heart (Vd estimated at 2 L/kg). After several hours of infusion, the Vd increases as concentrations in the blood equilibrate with the rapid equilibrating peripheral compartment (estimated Vd is 10 L/kg). There is delayed distribution to the slowly equilibrating deep peripheral compartment, which contains adipose tissue (Vd =60 L/kg). This takes up to 10 days to saturate this compartment. As Vd increases in this model with longer infusion time, elimination time also increases, from about 2 minutes to 70 minutes to about 1,400 minutes (5). It has been hypothesized that the excess lipid tissue in obesity would act as a large “sink”, prolonging time to arousal and potentially increasing level-dependent side effects (7).
Studies observing or recording alterations in clinical endpoints related to propofol infusion in obese patients are uncommon. Studies including the use of prolonged sedation using propofol in the ICU included non-obese patients with a mean duration of sedation from 12 to 99 hours (10-12). Most sedation trials do not report patient’s weight or BMI; in the few investigations when subjects were classified as obese, the majority looked at relatively short-term sedation in the setting of bariatric surgery (13). In our retrospective chart review we sought to better define the dose and effect relationships for this commonly used drug in the obese patient population receiving infusions in the MICU.
Propofol at our institution is the main sedative used for most patients entering the MICU. RASS scores are evaluated at least every nursing shift by the bedside nurse and documented into the electronic medical record and daily spontaneous awakening trials are performed. We found that the per kilogram actual body weight doses of propofol to reach goal RASS were lower in obese patients and progressively lower infusion rates were needed to maintain sedation in this group over time. Obese patients needed a median initial rate of 20 vs. 30 µg/kg/min in the non-obese in order to meet goal RASS. This phenomenon likely results from the higher total infused drug (based on excess adipose weight) rapidly penetrating the central compartment (and brain) to induce sedation with initial dosing (5). This finding suggests that potentially, initial infusion rates of propofol based on actual body weight in obese patients may be overestimated as compared to patients of normal weight to target a RASS of −2. Usage of other weights such as adjusted body weight for dosing must be further analyzed by future studies. When separating doses to achieve RASS scores of 0, −1, −2, and −3 during the first 5 days of infusion, we also found that the infusion requirements in the obese population were lower in comparison with non-obese patients with deepening sedation (RASS of −3). This was most notable after 48 hours, although a true correlation and any solid conclusions from this data is hard to discern.
Our results showing that obese patients needed progressively decreasing maintenance infusion rates, shown in Figure 2 is more challenging to explain. The Vd for propofol is heavily influenced by the duration of the infusion as predicted by the three-compartment model of distribution. Despite the quoted length of time for equilibration within adipose tissue, movement of drug outside of this deep compartment back into the central circulation may be quicker than appreciated; thus, possibly providing a readily available reservoir to maintain sedative doses of propofol in the brain. Alternatively, once the deep tissue compartments are saturated, continued infused drug may exclusively remain in the central compartment to maintain sedation. This may explain the observed decrease in per kilogram requirement in this patient population. About 90% of the patients enrolled in this study were on concomitant fentanyl. Fentanyl, a lipophilic drug used predominantly for analgesia, could have additive CNS effects when co-infused with propofol. In this study it was used for pain control and was not titrated to a goal RASS scores. Additionally, infusion rates of fentanyl remained consistent over time in both groups, making its contribution to propofol dose variability unlikely.
Time to extubation after discontinuation of propofol infusions was not statistically significant between both groups, though the observed difference in time (7 hours in the obese vs. 2 hours in the non-obese, P=0.236) may be clinically relevant. This endpoint excluded patients who expired or who received tracheostomy for long-term ventilation (69% of patients included), reducing statistical power. Daily “wake-up” trials were not documented in this investigation due to the retrospective nature of the study, however if utilized, could potentially lessen the accumulation of propofol in adipose tissue leading to extubation times that are similar to non-obese patients. Future larger studies should assess this outcome variable; if truly longer in the obese population, the most likely explanation would be the adipose “sink” compartment, which could provide space for redistribution of drug out of the central compartment more rapidly once the infusion is discontinued, reducing CNS levels below the sedation threshold. Although some patients were continued on sedation after the discontinuation of propofol, there was no difference between the two groups in total sedation time (92 vs. 75 hours, P=0.641) or time on the mechanical ventilator (99 vs. 75 hours, P=0.799) in the obese group vs. the non-obese group, respectively.
In our study, hospital mortality was significantly greater in the obese group (56% vs. 31%, P=0.017). It is known that physical examination, imaging, medication dosing, nursing care, and airway management amongst many other treatment variables are more difficult to perform in patients with higher BMIs due to body habitus and lack of clinical data regarding medical management. However, our results of higher mortality are in contrast to current literature showing obese ICU patients in comparison to non-obese ICU patients have lower hospital mortality overall (14). Although the baseline APACHE II scores were similar between groups in our study, our findings of higher mortality may have limited applicability due to a multitude of confounding factors including co-morbidities, ICU complications, and barriers to care in obese patients as listed above.
This study did not find any differences in adverse events between the two groups. This could partly be due to the limited number of patients with data available for laboratory values such as triglycerides, creatinine kinase, and the availability of echocardiograms before and after the initiation of propofol. The low number of patients in this study and also the low number of patients with available data could have contributed to the non-significance found in identified adverse events. In our subgroup analysis of morbidly obese patients, the primary outcome had a similar trend to the original data, but we did not find a significant difference. The morbidly obese group also had a trend towards utilizing less propofol per day, but this was only significant on day 4. We also saw a trend towards longer time to extubation compared to the non-obese group, 24 vs. 2 hours (P=0.25). The differences found in this subgroup were not significant though statistical power was lacking.
To our knowledge, this study is the first study describing dosing requirement differences between obese and non-obese patients receiving greater than 24 hours of sedation with propofol infusions in the ICU. Though retrospective and small in sample size, the findings of lower per kilogram dosing requirements to achieve equal sedation in the obese group was statistically significant and can be explained by propofol’s pharmacokinetic profile. Similarly, the continuous downward trend in daily propofol requirements in obesity and our hypothesized explanation lends credence to this observation. The lack of robust differences between morbidly obese and obese patients is likely explained by the paucity of patients and the use of 2 groups rather than having BMI as a continuous variable. Due to the retrospective nature, the data is also limited by the presence and accuracy of electronic chart documentation. The study endpoints and clinical correlates need to be examined in a larger, multi-centered prospective trial to make more definite conclusions.
Conclusions
In our study, the rate of propofol infusions, calculated by actual body weight, and titrated to a goal RASS score were lower in obese patients, and continued to decrease over the length of the infusion. Increased adverse effects were not observed in our study. Though the nature of our study limits the strength of these findings, our results can be explained by the accepted three-compartment pharmacokinetic model of propofol. Increases in adipose tissue create a large reservoir for accumulated propofol, which if dosed based on actual body weight, could predict decreased time to achieve adequate sedation upon initiation and prolong the time to arousal allowing extubation. There are many dosing challenges in obese patients and an increased understanding of drug disposition and dose response is needed. Further research is warranted to better define propofol’s disposition in critically ill obese patients and to better guide dosing and titration regimens.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by The NYULMC Institutional Review Board (No. s14-01821). The IRB granted exemption from the need for informed consent.
References
- Hogue CW Jr, Stearns JD, Colantuoni E, et al. The impact of obesity on outcomes after critical illness: a meta-analysis. Intensive Care Med 2009;35:1152-70. [Crossref] [PubMed]
- Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet 2000;39:215-31. [Crossref] [PubMed]
- La Colla L, Albertin A, La Colla G, et al. No adjustment vs. adjustment formula as input weight for propofol target-controlled infusion in morbidly obese patients. Eur J Anaesthesiol 2009;26:362-9. [Crossref] [PubMed]
- Devlin JW, Skrobik Y, Gélinas C, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med 2018;46:e825-73. [Crossref] [PubMed]
- Morgan DJ, Campbell GA, Crankshaw DP. Pharmacokinetics of propofol when given by intravenous infusion. Br J Clin Pharmacol 1990;30:144-8. [Crossref] [PubMed]
- Barr J, Egan TD, Sandoval NF, et al. Propofol dosing regimens for ICU sedation based upon an integrated pharmacokinetic-pharmacodynamic model. Anesthesiology 2001;95:324-33. [Crossref] [PubMed]
- Ingrande J, Lemmens HJ. Anesthetic Pharmacology and the Morbidly Obese Patient. Curr Anesthesiol Rep 2013;3:10-7. [Crossref] [PubMed]
- Servin F, Farinotti R, Haberer JP, et al. Propofol infusion for maintenance of anesthesia in morbidly obese patients receiving nitrous oxide. A clinical and pharmacokinetic study. Anesthesiology 1993;78:657-65. [Crossref] [PubMed]
- Ingrande J, Brodsky JB, Lemmens HJ. Lean body weight scalar for the anesthetic induction dose of propofol in morbidly obese subjects. Anesth Analg 2011;113:57-62. [Crossref] [PubMed]
- Weinbroum AA, Halpern P, Rudick V, et al. Midazolam versus propofol for long-term sedation in the ICU: a randomized prospective comparison. Intensive Care Med 1997;23:1258-63. [Crossref] [PubMed]
- Hall RI, Sandham D, Cardinal P, et al. Propofol vs midazolam for ICU sedation: a Canadian multicenter randomized trial. Chest 2001;119:1151-9. [Crossref] [PubMed]
- Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: patient and clinician perceptions. Br J Anaesth 2001;87:684-90. [Crossref] [PubMed]
- Ruokonen E, Parviainen I, Jakob SM, et al. Dexmedetomidine versus propofol/midazolam for long-term sedation during mechanical ventilation. Intensive Care Med 2009;35:282-90. [Crossref] [PubMed]
- Zhao Y, Li Z, Yang T, et al. Is body mass index associated with outcomes of mechanically ventilated adult patients in intensive critical units? A systematic review and meta-analysis. PLoS One 2018;13:e0198669. [Crossref] [PubMed]
Cite this article as: Johnson AL, Altshuler D, Schwartz DR, Papadopoulos J. Effect of obesity on propofol dosing requirements in mechanically ventilated patients in a medical intensive care unit. J Emerg Crit Care Med 2018;2:97.