Surveillance of community-acquired pneumonia in critically ill patients
Introduction
According to the statistics of Centre of Health Protection, pneumonia is the second most common cause of death in Hong Kong (1). Therefore, it poses heavy burden on the healthcare service, especially for the public hospitals, leading to significant resource implication in Hong Kong.
Community-acquired pneumonia (CAP) is a common cause for hospital admission. It was estimated that the annual incidence rate of CAP in adults was about 2.6 per 1,000 inhabitants with annual mortality rates about 0.1 per 1,000 inhabitants. The occurrence was more common in males and at the extreme ages of life. Between 22% and 51% of such patients were hospitalized for treatment (2). About 5% to 10% of hospitalized patients were more severely ill and managed in ICU. Moreover, up to 58% of those in ICU might eventually die of such infection (3-5).
Influenza and pneumococcus are two of the most important infective agents for CAP. Severe epidemics are commonly caused by influenza A and they may lead to loss of lives of many people. If influenza A and streptococcal infection coexist, the mortality rate is particularly high (6-11). During the “Spanish Flu” in 1918, it was estimated that it caused the death of 40 to 50 million people worldwide and many of them were due to co-infection. Subsequent pandemics in 1957 and 1968 also killed a lot of people though not to such extent. In 2009, a new strain of influenza A (H1N1) virus which had not ever been detected before, emerged and caused the 2009 H1N1 pandemic (6,7).
In this study, we aimed to investigate the epidemiology of severe CAP, including the causative agents, its risk factors, complications and outcomes of the disease in Hong Kong. It is envisaged that it will provide useful information for resource allocation to combat such disease.
Methods
Study design
This is a retrospective cohort study of patients with severe CAP admitted to an adult ICU in a tertiary hospital between January 2010 and December 2014. The medical records of patients with APACHE IV admission diagnosis of “viral pneumonia”, “bacterial pneumonia”, and “sepsis, pulmonary origin” within this period were identified. Community acquired pneumonia was defined as any chest infection diagnosed within the first 48 hours of hospitalization and infections occurring later were considered nosocomial. Also, patients were commonly admitted to ICU within 24 hours after the diagnosis of CAP was made. Therefore, those patients admitted to ICU 3 days after hospitalization were excluded as such infection might be hospital-acquired. The medical records of the patients were reviewed for data collection.
Data collected
The following patient data were extracted from the medical records: (I) demographic data, including age, sex, date of hospital and ICU admission, (II) comorbidities which are defined in the Chronic Health Evalution component of the APACHE IV model, including chronic obstructive airway disease, congestive heart failure, cirrhosis, chronic renal failure, diabetes mellitus, metastatic carcinoma, hematological malignancy and long term steroid therapy and (III) the APACHE IV scores.
All patients had microbiological investigations upon admission to ICU and the etiological diagnosis of CAP was determined for each patient as far as possible. Bacteria or viruses were identified by isolation and culture from blood, pleural fluid, sputum and endotracheal aspirate. Influenza A and B, parainfluenza, adenovirus, respiratory syncytial virus, Coxsackie virus, rhinovirus and human metapneumovirus antigens were detected in nasopharyngeal or endotracheal aspirate by immunofluorescence or real time PCR. Urinary samples were collected to test for Legionella and pneumococcal antigen. Paired serology for respiratory viruses, Mycoplasma and Chlamydia pneumoniae was collected on admission to ICU and 4 weeks later. Positive serological test was defined as a fourfold increase in antibody levels.
Staphylococcus aureus was considered as the etiological organism only if no other infective agents were isolated. When Staphylococcus aureus was grown from sputum or endotracheal aspirate, it was regarded as colonizers if other infective organisms were identified to cause the pneumonia.
Laboratory data within the first 24 hours of ICU admission, including white cell count, platelet count, C-reactive protein, creatinine level and bilirubin level were obtained from the medical records. The clinical notes were also reviewed for use of invasive mechanical ventilation, vasopressor drugs, renal replacement therapy and other advanced modes of ventilatory support, including inhaled nitric oxide (NO) and ECMO within 48 hours of ICU admission. Outcomes of patients, in terms of mortality and length of stay, in ICU and hospital were also extracted from the medical records.
Statistical analysis
Discrete variables were expressed as counts (percentage) and continuous variables as means ± SD. Univariate analyses were performed to detect association of outcomes with the demographic and clinical characteristics of the patients. Moreover, the causative agents of the infection were analysed. Differences between groups were assessed using the chi-square test and the Fisher exact test for categorical variable and the Student t test or Mann-Whitney U test for continuous variables. Multivariate regression analysis was used to assess the impact of independent variables on complications and outcomes. We performed multivariate logistic regression analysis to identify factors independently associated with in-hospital mortality, with the multivariate model constructed by using both stepwise-selection and backward elimination technique.
Results
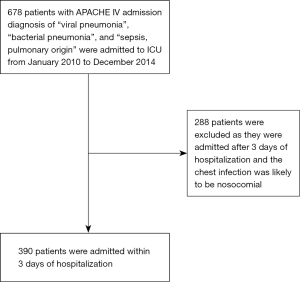
Totally 678 patients were identified to be admitted to our ICU for pneumonia from 2010 to 2014. From the cohort, 390 of them were admitted to ICU within 3 days of hospitalization (Figure 1).
Clinical features
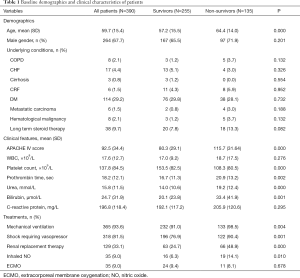
Demographic data and clinical characteristics of patients were shown in Table 1. Their age ranged from 18 to 91 years old and there was male preponderance. The severity of illness is reflected by the APACHE IV score.
Full table
Most of the patients had no chronic medical problems and the most common comorbidity was diabetes mellitus. Moreover, C-reactive protein level was marked elevated with mean of almost 200 mg/dL. More than three quarters of the patients required invasive mechanical ventilatory support while more than 80% needed vasopressors for hemodynamic support. About one third of them developed renal failure with institution of renal replacement therapy. However, nitric oxide and ECMO were uncommonly employed for support of such patients.
Microbiological data
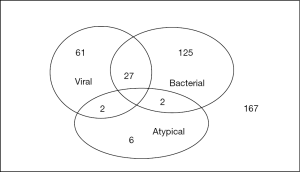
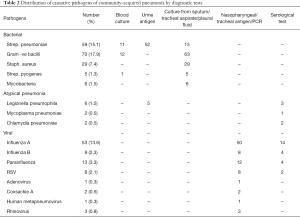
We were able to obtain the pathogens responsible for the CAP in about 60% of patients and the distribution is shown in Table 2 and Figure 2. Bacterial infection was identified in 154 patients and 27 of them suffered from co-infection of both bacteria and viruses. Streptococcus pneumoniae was the most commonly isolated organism and accounted for almost 40% of the bacterial pneumonia. It was detected by one or more of the following methods: blood culture, sputum/endotracheal aspirate/pleural fluid culture or urine antigen test. Gram-negative organisms constituted about 45% of bacterial chest infection. The common agents included Pseudomonas aeruginosa, Klebsiella pneumoniae and Acinetobacter baumannii. Other bacterial organisms identified were Staphylococcus aureus, Streptococcus pyogenes and Mycobacterium tuberculosis. Out of the 154 patients, 15 of them suffered from infection of more than 1 bacterium.
Full table
Atypical pneumonia only contributed to a minority of CAP of about 2%. Legionella pneumophila was the most common organism which was frequently detected by urine antigen test.
Viral pneumonia was detected in 88 patients and the causative virus was identified by antigen or PCR from nasopharyngeal aspirate or endotracheal aspirate as well as serological test. Moreover, coinfection of bacteria and viruses occurred in about one-third of such cases. Approximately 70% of viral infection was caused by influenza A, followed by influenza B and parainfluenza.
Outcome and prognostic factors
The overall ICU and hospital mortality rate was 23.1% and 34.6%, respectively, with mean ICU stay of 11.3±12.2 days and mean hospital stay of 28.7±51.7 days. The hospital survivors had shorter ICU length of stay (9.9±9.7 vs. 13.9±15.6 days, P=0.008) and hospital length of stay (26.8±25.9 vs. 32.1±80.5 days, P=0.457).
In univariate analysis, clinical variables associated with mortality included age, APACHE IV score, platelet count, prothrombin time, urea, bilirubin, need of mechanical ventilation, shock requiring vasopressor support, need of renal replacement therapy and inhaled NO therapy. C-reactive protein was not found to be associated with mortality of the patients.
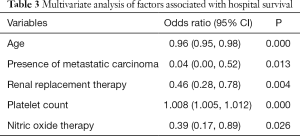
In order to identify independent prognostic factors, we performed multivariate analysis that included significant variables from the univariate analysis. Because the APACHE score was calculated from some of the independent variables, it was not included in the final model. Significant factors identified to be associated with mortality included age, platelet count, presence of metastatic carcinoma, need of renal replacement therapy and use of nitric oxide therapy (Table 3).
Full table
The mortality rates for CAP caused by bacteria, viruses and atypical organisms were 44.3%, 24.4% and 10.0%, respectively (P<0.05). The mortality rate of those with no identifiable organisms was 32.2%. Among those with bacterial infection, the mortality rates of pneumonia caused by Streptococcus pneumoniae, Gram negative bacilli and Staphylococcus aureus were 40.7%, 44.3% and 55.2%, respectively (P=0.433). For the viral infection, the mortality rates of pneumonia caused by influenza A, influenza B and parainfluenza infection were 18.9%, 55.6% and 30.8%, respectively (P=0.057).
Discussion
In this study, we reported the largest cohort of severe CAP in Hong Kong. The annual admission of our ICU was around 1,000. There were about 100 cases of severe CAP admitted to our ICU each year and it accounted for about 10% of our admission.
The causative agents of pneumonia were identified in 60% of cases in the present study. Streptococcus pneumoniae was the most common bacterial pathogen of CAP in our cohort, contributing to about 15% of the CAP. The mortality rate was about 40%, which was similar to that reported by other publications (5,12,13). In some studies, it was found to be associated with early death, probably due to early inflammatory process (14). Associated factors of early death included age, septic shock and inadequate empirical antibiotic treatment but pneumococcal bacteremia was not found to be associated with a worse prognosis in some studies (3,15).
Gram negative bacilli were also found to be common causative agents of severe CAP (12,13). In our study, about 20% were caused by Gram negative bacilli. It might be related to association with underlying lung conditions, such as bronchiectasis, in which Pseudomonas aeruginosa is a common causative agent of chest infection (16). Atypical pathogens, including Legionella pneumoniae, Mycoplasma and Chlamydia, were identified as the second most common causes of CAP in several studies (17-20) but it was not found in the present cohort. Most of the studies were conducted in the Western countries and geographical variation may account for such difference. Also, it was found that the prognosis of atypical pneumonia in our cohort had a relatively favourable outcome compared to bacterial and viral infection. In about 40% of our patients, no etiological organisms could be identified and it might be related to previous antibiotic usage.
Viral pneumonia is becoming more important especially during influenza epidemics (21-23). The most common agent is influenza A and other respiratory viruses include influenza B, parainfluenza, rhinovirus, respiratory syncytial virus, Coxsackie virus, human metapneumovirus and adenovirus. Viral agents were identified as causative organisms in about 20% of CAP in our study. The most common virus was influenza A, followed by parainfluenza and influenza B. In some studies, the infected patients were found to be younger and had a higher body mass index. Viral pneumonia was usually associated with a relatively good prognosis and the overall mortality rate was lower than that of bacterial pneumonia (7,23). In our study, the mortality rate of viral pneumonia was about 25%. Influenza A infection was found to have a better outcome than that of influenza B but it was not statistically significant.
In our cohort, the overall ICU mortality was 23% which was lower than that of other studies (12-14,24). One of the possible reasons for the improvement in the outcome may be due to advances in the clinical practice and medical technologies, such as use of ECMO. Moreover, it was found that underlying comorbidities did not affect the outcome of our patients except presence of metastatic carcinoma. In contrast, age, low platelet count, early development of acute kidney injury necessitating renal replacement therapy and need of nitric oxide therapy adversely affected their outcome.
In our study, pneumococcus and influenza were major causes of severe CAP. It was suggested that people infected by influenza may be prone to bacterial chest infection (9-11). Therefore, pneumococcal and influenza vaccination may be an effective means to reduce hospitalisation and death due to chest infection.
Pneumococcal vaccines are available in many developed countries. In USA, Centres for Disease Control and Prevention (CDC) recommends pneumococcal polysaccharide vaccine for all adults 65 years or older, people 2 through 64 years old with certain medical conditions, and adults 19 through 64 years old who smoke cigarettes (25). Also, CDC recommends routine annual influenza vaccination for all persons aged older than 6 months who do not have contraindications (26).
In Hong Kong, pneumococcal vaccines have been provided to the high risk group by the government. Elders aged 65 years or above and persons in the high-risk groups should also get pneumococcal vaccination for personal protection (27). The high risk groups include those with history of invasive pneumococcal disease (IPD), immunocompromised state, chronic medical diseases and cochlear implants. In Hong Kong, the annual incidence of IPD ranged from 1.7 to 2.9 per 100,000 between 2007 and 2015. The incidence is higher in children younger than 5 years old and adults old than 65 years. In addition, the Scientific Committee on Vaccine Preventable Diseases recommends seasonal influenza vaccination for high risk groups such as pregnant women, elderly persons living in residential care homes, persons with chronic medical problems, health care workers, children between the age of 6 months to 11 years, poultry workers and pig farmers and pig-slaughtering industry personnel. It is hoped that such vaccination programme can improve the outcome of high-risk patients (28).
There are several limitations of our analysis. First, it is a retrospective rather than prospective randomized study and certain biases may be introduced. Second, the treatment strategies, such as antibiotic treatment, ventilation protocol and renal support are not standardized. Therefore, it was difficult to analyse the effect of the treatment on patients’ outcome. Third, the serotypes of Streptococcus pneumoniae were unknown and certain strains may have poorer prognosis (29). Therefore, the impact of different strains of streptococcal infection could not be analysed accordingly.
Conclusions
CAP constituted about 10% of admission in our ICU. The common etiological organisms included Streptococcus pneumoniae and Influenza A. Pneumococcal and influenza immunizations may be effective to reduce the incidence of CAP. Such vaccine should be provided to the high risk group so that it may improve the outcome of severe chest infection.
Acknowledgements
This research was supported by funding from the Kowloon Central Cluster Research Grant of Hong Kong Hospital Authority.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Research Ethics Committee (KC/KE) (KC/KE-12-0161/ER-3).
References
- Death Rates by Leading Causes of Death, 2001 - 2017. Available online: https://www.chp.gov.hk/en/statistics/data/10/27/117.html
- Almirall J, Bolíbar I, Vidal J, et al. Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur Respir J 2000;15:757-63. [Crossref] [PubMed]
- Woodhead M, Welch CA, Harrison DA, et al. Community-acquired pneumonia on the intensive care unit: secondary analysis of 17,869 cases in the ICNARC Case Mix Programme Database. Crit Care 2006;10 Suppl 2:S1. [Crossref] [PubMed]
- Alkhayer M, Jenkins PF, Harrison BDW. The outcome of community acquired pneumonia treated on the intensive care unit. Respir Med 1990;84:13-6. [Crossref] [PubMed]
- Leroy O, Santre C, Beuscart C, et al. A five-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med 1995;21:24-31. [Crossref] [PubMed]
- Bertolini G, Rossi C, Crespi D, et al. Is influenza A (H1N1) pneumonia more severe than other community-acquired pneumonias? Results of the GiViTL survey of 155 Italian ICUs. Intensive Care Med 2011;37:1746-55. [Crossref] [PubMed]
- Martín-Loeches I, Sanchez-Corral A, Diaz E, et al. Community-acquired respiratory coinfection in critically ill patients with pandemic 2009 influenza A (H1N1) virus. Chest 2011;139:555-62. [Crossref] [PubMed]
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/ American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44:S27-S72. [Crossref] [PubMed]
- McCullers JA, Regh JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis 2002;186:341-50. [Crossref] [PubMed]
- Plotkowski MC, Puchelle E, Beck G, et al. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am Rev Respir Dis 1986;134:1040-4. [Crossref] [PubMed]
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289:179-86. [Crossref] [PubMed]
- Woodhead MA, Macfarlane JT, Rodgers FG, et al. Aetiology and outcome of severe community-acquired pneumonia. J Infect 1985;10:204-10. [Crossref] [PubMed]
- Walden AP, Clarke GM, McKechnie S, et al. Patients with community acquired pneumonia admitted to European intensive care units: an epidemiological survey of the GenOSept cohort. Crit Care 2014;18:R58. [Crossref] [PubMed]
- Mongardon N, Max A, Bouglé A, et al. Epidemiology and outcome of severe pneumococcal pneumonia admitted to intensive care unit: a multicenter study. Crit Care 2012;16:R155. [Crossref] [PubMed]
- Hirani NA, Macfarlane JT. Impact of management guidelines on the outcome of severe community-acquired pneumonia. Thorax 1997;52:17-21. [Crossref] [PubMed]
- Torres A, Serra-Batlles J, Ferrer A, et al. Severe community-acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis 1991;144:312-8. [Crossref] [PubMed]
- Moine P, Vercken JB, Chevret S, et al. Severe community-acquired pneumonia. Etiology, epidemiology, and prognosis factors. French Study Group for Community-Acquired Pneumonia in the Intensive Care Unit. Chest 1994;105:1487-95. [Crossref] [PubMed]
- Ruiz M, Ewig S, Torres A, et al. Severe community-acquired pneumonia. Risk factors and follow-up epidemiology. Am J Respir Crit Care Med 1999;160:923-9. [Crossref] [PubMed]
- Rello J, Quintana E, Ausina V, et al. A three-year study of severe community-acquired pneumonia with emphasis on outcome. Chest 1993;103:232-5. [Crossref] [PubMed]
- Pachon J, Prados MD, Capote F, et al. Severe community-acquired pneumonia. Etiology, prognosis, and treatment. Am Rev Respir Dis 1990;142:369-73. [Crossref] [PubMed]
- Zarychanski R, Stuart TL, Kumar A, et al. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ 2010;182:257-64. [Crossref] [PubMed]
- Patel M, Dennis A, Flutter C, et al. Pandemic (H1N1) 2009 influenza. Br J Anaesth 2010;104:128-42. [Crossref] [PubMed]
- ANZIC Influenza Investigators, Webb SA, Pettilä V, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 2009;361:1925-34. [Crossref] [PubMed]
- Gowardman J, Trent L. Severe community acquired pneumonia: a one-year analysis in a tertiary referral intensive care unit. N Z Med J 2000;113:161-4. [PubMed]
- Pneumococcal Vaccination. Available online: https://www.cdc.gov/vaccines/vpd/pneumo/index.html
- Seasonal Influenza (Flu) Vaccination and & Preventable Disease. Available online: https://www.cdc.gov/vaccines/vpd/flu/index.html
- Frequently Asked Question on pneumococcal vaccine. Available online: https://www.chp.gov.hk/en/features/26868.html
- Frequently Asked Questions on Seasonal Influenza Vaccine. 2017/18. Available online: https://www.chp.gov.hk/en/features/49312.html
- Mizrachi-Nebenzahl Y, Lifshitz S, Teitelbaum R, et al. Differential activation of the immune system by virulent Streptococcus pneumoniae strains determines recovery or death of the host. Clin Exp Immunol 2003;134:23-31. [Crossref] [PubMed]
Cite this article as: Lam KW. Surveillance of community-acquired pneumonia in critically ill patients. J Emerg Crit Care Med 2019;3:1.