
Veno-arterial extracorporeal membrane oxygenation and prolonged use of mechanical compression device combine for good outcome after hypothermic cardiac arrest: a case report
Introduction
The use of automatic mechanical devices to deliver compressions during cardiac arrest is becoming widespread, and the data surrounding extracorporeal membrane oxygenation (ECMO) in this setting is similarly growing (1,2). The need for additional mechanical support to unload the left ventricle (LV) during peripheral ECMO is also well-established (3). Herein, we report a case of hypothermic cardiac arrest requiring veno-arterial (VA) ECMO in which LV unloading was necessary but endovascular mechanical devices could not be placed. Concomitant, prolonged use of an external mechanical compression device along with VA ECMO facilitated a positive neurologic and clinical outcome.
Case presentation
A 38-year-old male (height 184 cm, weight 225 kg, BMI 66.5) with a history of gastroesophageal reflux disease, hypertriglyceridemia, alcohol dependence, depression, morbid obesity and previous suicide attempts was admitted to the emergency department (ED) via ambulance after being found on a riverbank with only his lower legs submerged in the water. It was winter and the average outside temperature was 4 degrees Celsius (39.2 Fahrenheit). The length of his exposure and whether there had been any near drowning or traumatic injury was unknown. The last known contact with the patient was approximately 5 hours prior to being found by passers-by.
On assessment by EMS he was unconscious, had agonal breaths and was in asystole. Assisted ventilation with bag-valve mask and CPR were immediately instituted. On admission to the ED he had a Glasgow Coma score (GCS) of 3, agonal breathing, remained in asystole with ongoing CPR, and was hypothermic at 27.4 degrees Celsius. He was intubated and a LUCAS chest compression system (Jolife, Lund, Sweden) was placed and initiated. A hospital ‘Shock Team’ call was initiated, a cervical collar was placed, and a transesophageal echo (TEE) was performed while spinal precautions were instituted. (The hospital ‘Shock Team’ protocol activates a telephone messaging service for cardiothoracic critical care, advanced heart failure, cardiothoracic surgery, an ECMO nurse specialist, cardiac anesthesia, and echocardiography to rapidly develop and initiate a mechanical support strategy.)
The TEE showed cardiac standstill, no pericardial effusion or valvular abnormalities, and suboptimal placement of the LUCAS device. The device was placed slightly too high on the chest and was compressing the ascending aorta. The device was repositioned under TEE guidance until it was effectively compressing the LV without obstructing the outflow tract (Figure 1). The ‘Shock Team’ decision was made to place him on a VA ECMO circuit to support his cardiovascular system and allow for rewarming to normothermia.
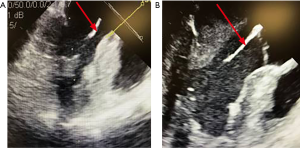
At this stage, known downtime was a total of 35 minutes. Initial blood work revealed a pH of 7.09, lactate of 3.7, base excess of −7.0, and a pO2 of 63 mmHg. Venous and arterial cannulations were performed via the left femoral approach. The patient’s body habitus, difficult anatomy, and hypothermia precluded percutaneous cannulation and thus a cut-down was performed. Further complicating cannulation was his apparent disseminated intravascular coagulopathy (DIC), though initial blood testing revealed hemoglobin level of 13.7 g/dL, platelets of 260 and international normalized ratio of 1.4. A subsequent left femoral arterial injury required emergent vascular surgery repair and movement of venous and arterial ECMO cannulas to the right femoral vein and artery. Ultimately, due to the various obstacles described, institution of VA ECMO took a total of 70 minutes, bringing the total known downtime to 105 minutes.
During this time he received multiple medications including sodium bicarbonate, calcium chloride, epinephrine, norepinephrine, vasopressin, vancomycin, piperacillin-tazobactam, fentanyl, and propofol. The LUCAS device provided 80 minutes of CPR during resuscitation and institution of VA ECMO, which was now delivering 5.5 L of flow per minute retrograde into the femoral artery. TEE re-evaluation post ECMO cannulation showed continued cardiac standstill and no aortic valve opening despite aggressive use of continuous epinephrine and milrinone.
The need for ongoing mechanical LV unloading was obvious. However, traditional endovascular devices like an intra-aortic balloon pump or an Impella were thought unusable, given the aforementioned vasoconstriction, acidosis, coagulopathy, BMI of 66, and overall poor target vessels as evidenced by the difficulty in placing the ECMO cannulas. Thus, the decision was made to continue utilizing the LUCAS device to assist in venting the LV. Position of the LUCAS device was once again optimized using TEE to maximize compression of the LV without obstructing the outflow tract.
During the prolonged time to institute cardiac support his acid-base status and coagulation parameters had deteriorated significantly: pH 6.97, bicarbonate 10mmol/L, base excess −21.8, INR 2.3, platelets 138,000/µL, and fibrinogen <80 mg/dL. We continued cardiovascular hemodynamic support with VA ECMO and the LUCAS device while rewarming to provide the most optimal setting for cardiac and neurologic recovery. Continuous renal replacement therapy was instituted from the ECMO circuit to facilitate normalization of the acid-base status.
The patient was deliberately and slowly rewarmed, 0.5 to 1 degree Celsius per hour, using the warmer on the ECMO circuit. After 6 hours and 20 minutes of VA ECMO support, concomitant Lucas device support (a total of 460 minutes using the Lucas device), and gradual rewarming, the patient had a spontaneous change of rhythm at 35.6 degrees Celsius to ventricular fibrillation. We performed a single DC-cardioversion shock at 200 J which converted him to a sinus tachycardia. A repeat TEE now showed an ejection fraction of 10% after ceasing the activity of the LUCAS. We continued to warm to normothermia using VA ECMO. Over the next 16 hours his coagulopathy and acid-base status improved. As we now had some clinical stability we performed a full body CT to rule out any traumatic injury and it was entirely negative. We then transferred him to the operating room for placement of a right axillary Impella CP for left ventricular decompression, a left-sided lower extremity distal perfusion cannula, and bilateral four compartment fasciotomies for a raised creatinine kinase of 18,284 U/L and reduced peripheral pulses of the lower limbs.
His head CT was normal as was continuous EEG over a period of 48 hours. Normal circuit anticoagulation was instituted to prevent complications. Over the next 96 hours we made significant reductions in inotropic and vasopressor requirements and TEE showed significant recovery of the biventricular function to normal. The Impella CP was removed 7 days after insertion following a weaning protocol. VA ECMO support was weaned over the ensuing 4 days and it was removed 12 days after initiation with full recovery of biventricular function. Over the next three days he was weaned from mechanical ventilation and extubated. He was transferred out of the ICU on hospital day 18 and underwent a right-side above-the-knee amputation on hospital day 22, the unfortunate ultimate result of vascular/perfusion injury sustained during his mechanical support course. He ultimately discharged to a rehabilitation center on day 34 and returned home 56 days after initial presentation.
Discussion
The use of extracorporeal and mechanical support during cardiac arrest has been well-described (4,5). Indeed, the 2015 American Heart Association guidelines for cardiopulmonary resuscitation discuss extracorporeal-CPR (ECPR) and “mechanical piston devices” as potential therapeutic options in certain cardiac arrest scenarios, including hypothermia (6). Asystolic, hypothermic arrest is perhaps the most unique niche in which ECPR can be applied, as additional mechanical LV unloading will be almost universally required in the setting of peripheral ECMO. In our literature search, we were unable to find another report describing such extended use of a mechanical compression device (such as the LUCAS device) to facilitate left ventricular unloading.
The LUCAS device has been described as an effective compression device while ECMO is being initiated (7), and centers have begun protocolizing the combined use of both modalities during ECPR (8). Prolonged use of the LUCAS device (without ECMO) during cardiac arrest is described, with the longest run time we could find being 2 hours and 45 minutes (9,10). In this case, the LUCAS device was utilized continuously for nearly 8 hours while the patient was cautiously rewarmed.
Another important highlight of this case is the use of TEE to optimize LUCAS device position, as the literature supporting this practice is also scant. Giraud, et al describe a case of ineffective chest compressions as well as right heart myocardial damage thought related to the LUCAS (11). Indeed in our case, before the LUCAS device was visualized and repositioned under TEE guidance, the aorta was being compressed with every beat, thus minimizing (if not entirely negating) the intended offloading effect.
While the LUCAS device played a pivotal role in this successful case, the use of mechanical compression devices is not without its reported complications. Lung, rib, and visceral trauma have all been described in the literature, and more recent trials have failed to demonstrate a survival benefit compared to manual CPR (12,13). A recent randomized non-inferiority trial including 122 LUCAS device patients did not find significantly more serious visceral damage than manual CPR (14). In the case described here, manual compressions would likely not have been sustainable for such a prolonged period.
Conclusions
Prolonged LV unloading using an external compression device (LUCAS) in the setting of peripheral VA ECMO is feasible and was necessary while correcting hypothermia, coagulopathy, and acid-base abnormalities in this case. TEE guidance was critical to optimizing LUCAS position on the chest. The combined support allowed for return of spontaneous circulation and a successful neurologic outcome despite a downtime of greater than 100 minutes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Remino C, Baronio M, Pellegrini N, et al. Automatic and manual devices for cardiopulmonary resuscitation: A review. Adv Mechanical Eng 2018;10:1-14. [Crossref]
- Tan BK. Extracorporeal Membrane Oxygenation in Cardiac Arrest. Singapore Medical Journal 2017;58:446-8. [Crossref] [PubMed]
- Centofanti P, Attisani M, La Torre M, et al. Left Ventricular Unloading during Peripheral Extracorporeal Membrane Oxygenator Support: A Bridge To Life In Profound Cardiogenic Shock. J Extra Corpor Technol 2017;49:201-5. [PubMed]
- Lee JJ, Han SJ, Kim HS, et al. Out-of-hospital cardiac arrest patients treated with cardiopulmonary resuscitation using extracorporeal resuscitation using extracorporeal membrane oxygenation: focus on survival rate and neurologic outcome. Scand J Trauma Resusc Emerg Med 2016;24:74. [Crossref] [PubMed]
- Kagawa E. Extracorporeal cardiopulmonary resuscitation for adult cardiac arrest patients. World J Crit Care Med 2012;1:46-9. [Crossref] [PubMed]
- Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S444. [Crossref] [PubMed]
- Menegazzi JJ, Salcido DD, Housler GJ, et al. Feasibility of initiating extracorporeal life support during mechanical chest compression CPR: A Porcine Pilot Study. Resuscitation 2012;83:130-3. [Crossref] [PubMed]
- Yannopoulos D, Bartos JA, Martin C, et al. Minnesota Resuscitation Consortium’s Advanced Perfusion and Reperfusion Cardiac Life Support Strategy for Out-of-Hospital Refractory Ventricular Fibrillation. J Am Heart Assoc 2016;5. [PubMed]
- Matevossian E, Doll D, Säckl J, et al. Prolonged closed cardiac massage using LUCAS device in out-of-hospital cardiac arrest with prolonged transport time. Open Access Emerg Med 2009;1:1-4. [Crossref] [PubMed]
- Holley J, Ornato J, Heightman A. Mechanical CPR is producing resuscitation results beyond expectations. JEMS 25 Oct 2014. Web accessed 21 Aug 2018.
- Giraud R, Siegenthaler N, Schussler O, et al. The LUCAS 2 chest compression device is not always efficient: an echographic confirmation. Ann Emerg Med 2015;65:23-6. [Crossref] [PubMed]
- Wik L, Olsen JA, Persse D, et al. Manual vs. integrated automatic load-distributing band CPR with equal survival after out of hospital cardiac arrest. The randomized CIRC trial. Resuscitation 2014;85:741-8. [Crossref] [PubMed]
- Rubertsson S, Lindgren E, Smekal D, et al. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in out-of-hospital cardiac arrest. JAMA 2014;311:53-61. [PubMed]
- Koster RW, Beene LF, van der Boom EB, et al. Safety of mechanical chest compression devices AutoPulse and LUCAS in cardiac arrest: a randomized clinical trial for non-inferiority. Eur Heart J 2017;38:3006-13. [Crossref] [PubMed]
Cite this article as: Trethowan B, Michaud CJ, Kumar A, Strotbaum A, Berjaoui W, Spurlock D. Veno-arterial extracorporeal membrane oxygenation and prolonged use of mechanical compression device combine for good outcome after hypothermic cardiac arrest: a case report. J Emerg Crit Care Med 2019;3:4.


