
Nothing to sneeze at: tension pneumocephalus causing an acute stroke following endoscopic sinus surgery
Introduction
Pneumocephalus (PC) is defined as the abnormal collection of air within the cranial vault. This commonly results from violating the meninges by craniofacial trauma or surgery, infections and malignancy. The intracranial pneumocele typically self-dissipates and is only rarely clinically consequent (1). Conversely, tension PC (TPC) is a life-threatening condition wherein the mass-effect on the brain results in rapid neurological deterioration. The author describe a rare case of TPC in the early postoperative period following endoscopic sinus surgery (ESS) resulting in acute ischemic stroke. The TPC resolved by emergent decompression of the pneumocele resulting in near-complete recovery. The distinguishing features of TPC and its management are then discussed.
Case presentation
An 82-year-old male with a recent otolaryngologic procedure and a disclosed history of coronary artery disease, hypertension and chronic maxillary sinusitis, was transferred to our tertiary care center after suffering an acute change in mental status. On the day of admission, the patient underwent nasal sinus endoscopy for polypectomy and ethmoidectomy at an outside institution. The patient was discharged home post-operatively without immediate complications from the procedure. Approximately eight hours postoperatively from his ESS, he was discovered on the floor of his home with decreased responsiveness, word finding difficulty and generalized weakness. The family had also reported hearing a large sneeze just prior to this episode. He was urgently transported to the local community hospital wherein his vital signs were notable for an oxygen saturation of 80%, which normalized with a non-rebreather mask. The patient’s neurological exam was significant for an NIH Stroke Scale of 6 based on his dysarthria, aphasia, limb ataxia, and focal right leg weakness. A non-contrasted computed tomography of the head (NCHCT) was performed and revealed PC along frontotemporal convexities with mass-effect of adjacent brain parenchyma, consistent with TPC (Figure 1A,B,C) (2).
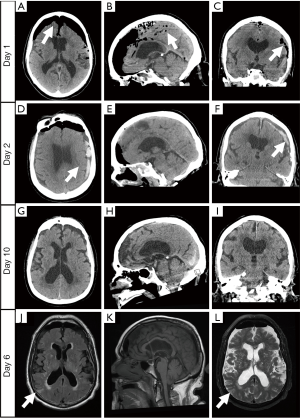
Upon arrival to our facility, the patient’s vital signs were only remarkable for a blood pressure of 197/97 mmHg. On primary survey, the patient had a Glasgow Coma Scale of 14. Pupils were midrange, equal and reactive. On secondary survey, there were no external signs of trauma, including any hemotympanum, CSF otorrhea or rhinorrhea. Initial laboratory studies showed a marked leukocytosis of 26,000 cells/mm3 and a lactic acid of 2.3 mmol/L. The patient’s complete metabolic profile was notable for a glucose of 308 mg/dL. A repeat NCHCT showed persistent TPC in the middle and anterior cranial fossa, with an air CSF level visible adjacent to the Sylvian fissure. The culprit defect was demonstrated in the left sphenoid sinus.
Based on these findings, neurosurgery was emergently consulted and the patient underwent an immediate left-sided burr-hole for decompression, which expressed air instantaneously. In the Neuro-Intensive Care Unit, a repeat NCHCT demonstrated near complete resolution of TPC. However, on hospital day (HD)-2 he developed a left-sided subdural hematoma with an associated midline shift (Figure 1D,E,F). The patient then underwent endoscopic exploration which did not readily demonstrate a repairable skull defect. Enhanced brain magnetic resonance imaging (MRI) on HD-6 revealed multiple acute ischemic strokes, likely secondary to the initial TPC (Figure 1G,H,I). The patient's neurological exam continued to improve through HD-10, wherein he was discharged to a rehabilitation facility with a Modified Rankin Scale of 2, consistent with minimal disability. The patient was seen in neurosurgery clinic at his 1-month follow up visit and was living independently with only mild disequilibrium.
Discussion
PC, also known as a pneumatocele or aerocele, is defined as abnormal entrapment of air within the cranial vault and can be extradural, subdural, subarachnoid, intraparenchymal and intraventricular. PC is a surrogate marker for the presence of an abnormal transcranial connection, which is often quite difficult to localize. The most common causes of PC are by craniofacial trauma, followed by neoplasm, spontaneous, iatrogenic, or by infectious spread (3,4). Other positive pressure events causing PC also include including coughing, sneezing, valsalva, and high altitude (5). Neurologic complications from ESS, including persistent CSF leak, post-operative hemorrhage, ascending meningitis, and pneumocephalus, are significantly rare, occurring in less than 1% of all cases (6). Two potential mechanisms have been described to explain the pathological basis leading to PC. The first proposed mechanism is the so-called “inverted soda bottle effect” (7). This involves the development of negative ICP from an ongoing CSF leak or drainage procedure, which leads to a vacuum effect to cause additional accumulation of air within the cranial cavity (8). This mechanism is particularly concerning given the presence of a persistent CSF-leak and pneumocephalus are associated with a risk of ascending meningitis in about 20–30% of cases (9,10). The other proposed mechanism, as in our case, involves a “ball-valve” mechanism creating a one-way entry of an “air tumor” in the cranial vault (11). The patient's history was complicated by a positive pressure event, a sneeze, wherein air was forced from the paranasal defect into the cranial cavity, without the possibility of egress. The anatomy of the frontal bone predisposes to development of pneumocephalus following violation of the particularly thin frontal and paranasal sinuses, as well fragile, and tightly adherent dural layer. Antibiotic prophylaxis is usually indicated due to development of an intracranial infection when persistent CSF leaks are observed (5). The management of pneumocephalus is mechanism-dependent. The majority of cases of pneumocephalus from small defects responds to conservative therapy and avoidance of barotrauma (5,12). Conservative measures include bed rest, head elevation, and avoidance of positive pressure, including nose-blowing, cough or valsalva (5). The addition of oxygen supplementation has also been shown to significantly reduce the pneumocele volume in post-craniotomy pneumocephalus (13). A recent study showed that the majority of skull defects following ESS may be as small as 3 mm and may respond to conservative therapy, whereas persistent defects are more predictive of the need for endoscopic repair (5). In the absence of persistent skull defects and ongoing CSF leak, antimicrobial therapy is controversial.
In most cases, PC is self-limited and patients typically do not experience symptoms. Any patient presenting with unexplained nausea, vomiting, headache, or altered mental status after ESS should undergo prompt CT imaging and surgical consultation for possible development of TPC. This entity remains a relatively rare and often clinically unrecognized etiology of cognitive dysfunction and neurological deterioration. Progressive air-trapping from a significant trans-cranial pressure differential can eventually result in vascular compromise. TPC is a both a clinical and radiographic diagnosis. CT imaging is the gold-standard diagnostic imaging modality for PC along with its complications. Radiographically, subdural TPC is demonstrated on a NCHCT by a “Mount Fuji sign”, with bilateral frontal lobe compression with interhemispheric separation, as seen in our case (Figure 1A) (2). The addition of T2-weighted MRI has also been shown to increase the detection of the skull defect, thus aiding in definitive repair. Signs and symptoms are similar to those seen with increased intracranial pressure (ICP) from space-occupying lesions, which include headache, altered mental status, focal neurologic deficits, convulsions, meningitis, and even herniation syndromes (1,4,5,14). Otolaryngology and neurosurgical consultation are usually indicated given definitive treatment of symptomatic cases involves emergent neurosurgical decompression to relieve the pressure on underlying brain parenchyma and repair of the culprit defect. Mannitol has also been reported to benefit in acutely lowering the ICP, however additional studies are required (14,15).
This case highlights a rare neurological presentation of ischemic stroke from TPC following barotrauma and recent ESS. To our knowledge, this is the first case of TPC resulting in acute ischemic stroke. Clinicians should always maintain a high index of suspicion for PC and its sequelae following trauma or even minor procedures. Headaches and abnormal mental status are the most common presenting clinical features of TPC, rather than lateralizing neurological deficits. Treatment involves prompt 100% oxygen administration, head of bed elevation and additional efforts to decrease the ICP and mass effect. As highlighted in this case, emergent surgical decompression of the space-occupying lesions and endoscopic exploration or craniotomy for definitive repair are also indicated to prevent progression of neurological deterioration and improve clinical outcomes (1,4,7,15).
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Schirmer CM, Heilman CB, Bhardwaj A. Pneumocephalus: case illustrations and review. Neurocrit Care 2010;13:152-8. [Crossref] [PubMed]
- Ishiwata Y, Fujitsu K, Sekino T, et al. Subdural tension pneumocephalus following surgery for chronic subdural hematoma. J Neurosurg 1988;68:58-61. [Crossref] [PubMed]
- Markham JW. The clinical features of pneumocephalus based upon a survey of 284 cases with report of 11 additional cases. Acta Neurochir (Wien) 1967;16:1-78. [Crossref] [PubMed]
- Andrews JC, Canalis RF. Otogenic pneumocephalus. Laryngoscope 1986;96:521-8. [Crossref] [PubMed]
- DelGaudio JM, Ingley AP. Treatment of pneumocephalus after endoscopic sinus and microscopic skull base surgery. Am J Otolaryngol 2010;31:226-30. [Crossref] [PubMed]
- Stankiewicz JA, Lal D, Connor M, et al. Complications in endoscopic sinus surgery for chronic rhinosinusitis: a 25-year experience. Laryngoscope 2011;121:2684-701. [Crossref] [PubMed]
- Lunsford LD, Maroon JC, Sheptak PE, et al. Subdural tension pneumocephalus. Report of two cases. J Neurosurg 1979;50:525-7. [Crossref] [PubMed]
- Horowitz M. Intracranial Pneumocoele. An Unusual Complication Following Mastoid Surgery. J Laryngol Otol 1964;78:128-34. [Crossref] [PubMed]
- Schoentgen C, Henaux PL, Godey B, et al. Management of post-traumatic cerebrospinal fluid (CSF) leak of anterior skull base: 10 years experience. Acta Otolaryngol 2013;133:944-50. [Crossref] [PubMed]
- Welch KC. Neurologic Complications and Treatment. Otolaryngol Clin North Am 2015;48:769-82. [Crossref] [PubMed]
- Dandy WE. Pneumocephalus (intracranial penumatocele or aerocele). Arch Surg 1926;12:949-82. [Crossref]
- Dabdoub CB, Salas G, Silveira Edo N, et al. Review of the management of pneumocephalus. Surg Neurol Int 2015;6:155. [Crossref] [PubMed]
- Gore PA, Maan H, Chang S, et al. Normobaric oxygen therapy strategies in the treatment of postcraniotomy pneumocephalus. J Neurosurg 2008;108:926-9. [Crossref] [PubMed]
- Nicholson B, Dhindsa H. Traumatic tension pneumocephalus after blunt head trauma and positive pressure ventilation. Prehosp Emerg Care 2010;14:499-504. [Crossref] [PubMed]
- L'Hommedieu LM, Dingeldein MW, Tomei KL, et al. Acute Management of Tension Pneumocephalus in a Pediatric Patient: A Case Report. J Emerg Med 2018;54:112-5. [Crossref] [PubMed]
Cite this article as: Cancelliere A. Nothing to sneeze at: tension pneumocephalus causing an acute stroke following endoscopic sinus surgery. J Emerg Crit Care Med 2019;3:16.


