Nursing in a critical care hyperbaric unit at Merida, Yucatan, Mexico: report of a case of an acute pediatric burn patient
Introduction
Hyperbaric medicine practice today, places special technical and knowledge-based skills demands on nurses, especially when handling intensive care unit (ICU) patients inside hyperbaric chambers (1). Probably the best clinical contributions of hyperbaric oxygen therapy (HBOT) are in the acute cases, in the ischemia reperfusion injury. Nevertheless in the last years, hyperbaric units have focused more in chronic wounds, do to the challenges of managing ICU patients inside a hyperbaric chamber.
Acute trauma in the world is a growing public health issue. Every year, 1.24 million people are killed in motor vehicle accidents. More than half of those killed on roads, are young adults aged between 15 and 44 years. It is estimated that there are around one hundred and forty thousand injured per day and half a million disabilities per year (2).
More than 90% of the road traffic deaths occur in low or middle-income countries. Trauma cost represents around 1% or 2% of the gross national product to these countries (3). The burden of trauma and other acute conditions should not be analyzed only with direct costs. We should also include indirect, and if possible, insensible costs. Then we will integrate the real global burden of disease.
Also, the burden and risks of injuries are different for economically developed and developing countries. In the later, there are more gaps in the chain of survival. Research is needed to understand the behavior of these injuries in a resource challenging setting (3). We need to conduct research and produce tropicalized evidence, to modify the actual local public health policies, in order to adjust them to the challenges of trauma in these countries.
Over the last few years there has been a reduction in the morbidity and mortality of burns in the world. Nevertheless, acute burn injury remains the leading cause of death in children ages 1 to 9 years (4). Most of the burns (68%) take place at home. Patients that experience a severe burn (>20% total body surface) are at high risk of developing infections. Patients with burns and trauma pose the greatest risk of morbidity and mortality (5). Also, 90% of deaths occur in economically developing countries (4-6). Direct burn cost ($3,000.00 to $5,000.00 USD per day), accounts only to the 23% of total cost (6).
The nurse in HBOT
The beginning of the participation of nurses in the hyperbaric environment dates to the first half of the 20th Century. One of the first examples took place in 1936 during the treatment of Lepromatous leprae with HBOT in Brazil with Dr. Osorio de Almeida. In the 1950’s with the use of hyperbaric chambers for pediatric cardiac surgeries performed in Holland by Dr. Ita Boerema, nurses ventured to work inside chambers but as surgical nurses (7,8).
Formal training in hyperbaric started at the end of the1960’s. At this time hyperbaric nurse training was similar to that of hyperbaric technicians. The preferred specialties for hyperbaric nurses were ICU and emergency care (8).
Many of the hyperbaric medicine departments depend on the leadership of their directors. The Memorial Medical Center at Long Beach was not the exception. Dr. George Hart inaugurated a hyperbaric medicine department with monoplace chambers, where Alice le Veille, a former US Navy nurse, trained hyperbaric nurses to develop a more important role during the treatments, especially when critical care patients were treated (8).
In the 1970’s, Diane Norkool leaded the movement of hyperbaric nurses at the Virgina Mason’s Hyperbaric Center, at Seattle, Washington. Hyperbaric nurse training continued to evolve and in 1985 they formed the Baromedical Nurse Association (BNA). Today they have members from almost all of the continents (8).
In the second half of the 1990’s several key events in hyperbaric nursing took place. First in 1995, the first publication of a hyperbaric nurse Chapter in Dr. Erik Kindwall’s Textbook on Hyperbaric Medicine Practice, came out. Second, The BNA Certification Board was established that same year. In 1998, the BNA published the standard of care for the patient receiving HBOT. The publication of the first hyperbaric nurse textbook was in 2002 (8).
In Mexico, hyperbaric medicine started in the early 1960’s with monoplace chambers at the Hospital “20 de Noviembre” with Michael C. Crist-Olivier and Penelope Luna de Gámez, who was trained in the United Kingdom (9). The first course of hyperbaric nursing was provided in 2001 at Hospital Ángeles del Pedregal in Mexico City. Now, Mexico has more than 600 hyperbaric units and several in important public and private hospitals, including institutes of health (7,9).
Since 2009, Judith Ruiz-Aguilar, RN, MEd, has taught the Diplomat on Nursing for Hyperbaric Oxygen Therapy without interruption, once a year in Merida, Yucatan. It was the first Diplomat to be certified by the Universidad Nacional Autónoma de México [UNAM (National Autonomous University of Mexico)]. Actually, UNAM is the only University to provide certified Diplomats for nurses, physicians and the only postgraduate course (one year). It has been an on going program since the year 2001. E. Cuauhtemoc Sanchez-Rodriguez started all of the courses.
In Yucatan the first hyperbaric medicine program was started in 1993 in the city of Tizimin. The chamber was predominantly, but not exclusively, used for the treatment of decompression illness in sea harvesters. The unit in Hospital Águstín O’Horan started in November of 2008. Since then, it has been the training center on hyperbaric medicine in Mexico. Presently, all of the courses are taught at Hospital Agustín O’Horan and the Centro de Especialidades Médicas del Sureste (CEM) in Merida, Yucatan.
HBOT in an ICU
The first golden rule to treat an ICU patient in a hyperbaric chamber is that you can maintain the same quality of care inside the chamber as in the ICU. This means that the hyperbaric unit has to have the technology means as well as the proficiency and competency of its personnel to provide intensive care inside the chamber.
To accomplish these, the unit needs to have nursing personnel competent in the use of supportive medical equipment, IV pumps, ventilators, ECG monitors, cardiac pacemakers, and defibrillators; as well as dealing with emergent patients during the HBOT.
According to the pathophysiology of the medical conditions where HBOT is used, it has two main indications: in the ischemic reperfusion event (acute conditions in the first 72 h) and in the chronic non-healing wounds. In order to get the maximum advantage of HBOT, we stress the need to treat conditions in the early stages (<6 hours).
HBOT in acute injuries has a double purpose. In the early stages (<72 h), it reduces the inflammatory response by modulating the cytokine and gene cascades (10,11). In the cellular phase of burn shock, the mitochondrial dysfunction creates a loss in ATP and a dysfunction of membrane ion pumps, ending in cytotoxic edema. Once there is mitochondrial dysfunction calcium is freed into the cytosol and becomes the first modulator of inflammation (12-15).
The inflammatory cascades generate oxidative stress and/or oxidative damage. Xanthine oxidase cascade generates more reactive oxygen species (ROS). The arachidonic acid cascade promotes the production of lipoxygenase, cyclooxygenase, thromboxane, prostaglandins and leukotrienes. In response to these and other mediators, there is an elevation of adhesion molecules, transcription factors, chemokines and endothelins (11,16) . The cytokine and gene cascades complement these responses (17-24).
HBOT reduces the production of thromboxane (A2 and B2), platelet-activating factor (PAF), integrin β2 and intercellular adhesion molecule 1 (ICAM-1). In the vascular, HBOT reduces the production of prostaglandins (PGE2), leukotrienes (LTB4 and D4), interleukins (TNF-α, IL-1β, IL-6, IL-8, and IL-10), and inducible nitric oxide synthase (iNOS) (11,16). In the late phases of burn treatment, HBOT is used to enhance the survival of grafts and flaps. The use of HBOT has proved to reduce hospital stay when used promptly (25,26).
According to the Undersea and Hyperbaric Medical Society (UHMS), and the American College of Hyperbaric Medicine (ACHM), thermal burns are an accepted condition. According to the Tenth European Consensus Conference of the European Committee for Hyperbaric Medicine (ECHM) of 2017, thermal burns are a type 2 recommendation with a level C evidence. The recommendation is to treat with HBOT, second degree burns with more that 20% body surface area (BSA). They also suggest that burns to the face (ear, nose), neck, hands, fingers and perineum may benefit even if the total surface burned is less than 20%. It is suggested that HBOT should be initiated within six to eight hours after the burn injury; and that two sessions per day be given for a minimum of three days (27,28).
Adjustments for the hyperbaric treatment in ICU pediatric patients
All the bed and patient clothing were changed for 100% cotton clothing and the patient used a ground wrist strap (Desco Co.), to avoid the possibility of electrostatic charges and sparks during HBOT. All the IV fluids were maintained as topside using the Baxter IV pumps and a hyperbaric IV extension kit (Argon Medical Devices). The sedation was maintained with midazolam and fentanyl with a continuous drip. It is important to remember that sedation under HBOT is more profound but lasts 30–40% less time than topside. In this case the ET tube did not have balloon so we did not need to exchange the air for water before pressuring the patient.
We did not perform myringotomies but we did pressurize and depressurize the patient 1 psi/min. Ventilated and sedated pediatric patients very rarely develop middle ear barotrauma due to the size and positioning of the Eustachian tubes and the lack of muscle resistance. Slow pressurization is a key factor to avoid this HBOT side effect. We maintained ECG and invasive blood pressure monitoring.
We used a TV and audio system with his favorite pictures during the treatment, even when he was sedated. One of the parents was always present at the unit during the HBOT treatment.
Case presentation
We present a case of a 5-year-old pediatric burn patient who suffered a deep second-degree burn that compromised 32% of the total body surface; including the face, neck, thorax and both arms. The source of the burns was direct flame.
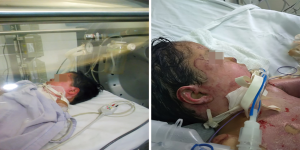
The patient was received at a social security hospital where he was stabilized and intubated, due to airway burn. Once stabilized and after 48 hours of the burn, the patient was referred to our hospital to continue management at the pediatric ICU and to start HBOT (Figure 1). The patient required IV fluid, norepinephrine, midazolam and fentanyl, during the first days of HBOT treatment. Wound care and debridement were performed daily and with the placement of gauzes with Triticum vulgare over the open wounds, which are compatible with oxygen at pressure.
Our hyperbaric unit has a 3300 Sechrist monoplace chamber that is equipped with a 500A Sechrist ventilator and five IV pumps (Baxter Flo-Gard 6201). We are able to monitor ECG and invasive arterial pressure. The treatment protocol used was 1.5 ATA for 60 minutes at pressure once a day. The patient was extubated after eight days, and did not developed ARDS. He received a total of 14 treatments once a day (QD), with complete healing of the burned areas (Figure 2). The total number of hospital stay was 17 days, which is lower than expected for a severe burned patient (average of 27.5±1.2 days) (28).
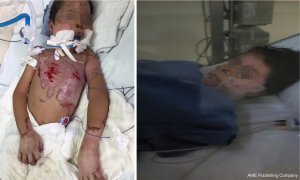
During the HBOT, the patient did not require any further surgical debridement in the operating room; all wound care was performed at bedside with a light sedation. The patient required IV antibiotic, but did not develop any infection. He was discharged finally to the social security hospital where he was sent home. He experienced neither secondary effects nor complications.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript.
References
- Ekeh A. Being-in-the-world of the trauma patient: A Heideggerian perspective. J Trauma Nurs 2016;23:173-6. [Crossref] [PubMed]
- World Health Organization. Global Status Report on Road Safety: Time for Action, 2009.
- World Health Organization. Global Status Report on Road Safety: Supporting a Decade of Action, 2013.
- Consunji R, Ameratunga S, Hyder AA. Trauma care in the developing world: Introduction to special issue. Surgery 2017;162:S2-3. [Crossref] [PubMed]
- Ellison DL. Burns. Crit Care Nurs Clin North Am 2013;25:273-85. [Crossref] [PubMed]
- World Health Organization. A WHO Plan for Burn Prevention and Care, 2008.
- Sanchez-Rodriguez C. Hyperbaric Medicine in Latin America. In: Jain KK. editor. Textbook of Hyperbaric Medicine, 6th edition. Switzerland, Springer, 2017:623-5.
- Larson-Lohr V. Nursing and Hyperbaric Medicine. In: Jain KK. editor. Textbook of Hyperbaric Medicine, 6th edition. Switzerland, Springer, 2017:511-22.
- García L, Sánchez EC. Terapia con oxigenación hiperbárica, conceptos básicos. Gaceta Médica de México 2000;136:45-56.
- Sehat A, Huebinger RM, Carlson DL, et al. Burn serum stimulates myoblast cell death associated with IL-6-induced mitochondrial fragmentation. Shock 2017;48:236-42. [Crossref] [PubMed]
- Mace JE, Park MS, Mora AG, et al. Differential expression of the immunoinflammatory response in trauma patients: burn vs no-burn. Burns 2012;38:599-606. [Crossref] [PubMed]
- Sánchez EC. Mechanisms of action of hyperbaric oxygenation in stroke. Crit Care Nurs Q 2013;36:290-8. [Crossref] [PubMed]
- Balderas E, Zhang J, Stefani E, et al. Mitochondrial BKCa channel. Front Physiol 2015;6:104. [Crossref] [PubMed]
- Vlodavsky E, Palzur E, Shehadeh M, et al. Post-traumatic cytotoxic edema is directly related to mitochondrial function. J Cereb Blood Flow Metab 2017;37:166-77. [Crossref] [PubMed]
- Tasoulis MK, Douzinas EE. Hypoxemic reperfusion of ischemic states: an alternative approach for the attenuation of oxidative stress mediated reperfusion injury. J Biomed Sci 2016;23:7. [Crossref] [PubMed]
- Matsuura H, Matsumoto H, Osuka A, et al. Clinical importance of a cytokine network in major burns. Shock 2019;51:185-93. [Crossref] [PubMed]
- Milovanovic M, Volarevic V, Radosvljevic G, et al. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res 2012;52:89-99. [Crossref] [PubMed]
- Sakuma S, Kitamura T, Kuroda C, et al. All-trans Arachidonic acid generates reactive oxygen species via xanthine dehydrogenase/ xanthine oxidase interconversion in the rat liver cytosol in vivo. J Clin Biochem Nutr 2012;51:55-60. [Crossref] [PubMed]
- Klein GL, Benjamin DA, Herndon DN. Calcemic response to burns differs between adults and children: review of the literature. Osteoporos Sarcopenia 2017;3:170-3. [Crossref] [PubMed]
- Weis A, Bohnert M. Expression patterns of adhesion molecules P-selectin, von Willebrand factor and PECAM-1 in lungs: a comparative study in cases of burn shock and hemorrhagic shock. Forensic Sci Int 2008;175:102-6. [Crossref] [PubMed]
- Lateef Z, Stuart G, Jones N, et al. The cutaneous inflammatory response to thermal burn injury in a Murine Model. Int J Mol Sci 2019. [Crossref] [PubMed]
- Zhang ZS, Chen W, Li T, et al. Organ-specific changes in vascular reactivity and roles of inducible nitric oxide synthase and Endothelin-1 in a rabbit endotoxic shock model. J Trauma Acute Care Surg 2018;85:725-33. [Crossref] [PubMed]
- Rae L, Fidler P, Gibran N. The physiologic basis of burn shock and the need for aggressive fluid resuscitation. Crit Care Clin 2016;32:491-505. [Crossref] [PubMed]
- Ogunbileje JO, Porter D, Chao T, et al. Hypermetabolism and hypercatabolism of skeletal muscle accompany mitochondrial stress following severe burn trauma. Am J Physiol Endocrinol Metab 2016;311:E436-48. [Crossref] [PubMed]
- Villanueva E, Bennett MH, Wasiak J, et al. Hyperbaric oxygen therapy for thermal burns. Cochrane Database Syst Rev 2004;3:CD004727. [PubMed]
- Gesell LB. Hyperbaric Oxygen Therapy Indications. Durham, NC: Undersea and Hyperbaric Medical Society, 2008.
- Mathieu D, Marroni A, Kot J. Consensus Conference. Tenth European Consensus Conference on Hyperbaric Medicine: recommendations for accepted and non-accepted clinical indications and practice of hyperbaric treatment. Diving Hyperb Med 2017;47:24-32. [Crossref] [PubMed]
- Koç Z, Saglam Z. Burn epidemiology and cost of medication in paediatric burn patients. Burns 2012;38:813-9. [Crossref] [PubMed]
Cite this article as: Ruiz-Aguilar J, Diaz-Ibañez R, Sanchez-Rodriguez EC. Nursing in a critical care hyperbaric unit at Merida, Yucatan, Mexico: report of a case of an acute pediatric burn patient. J Emerg Crit Care Med 2019;3:26.