Focused airway ultrasound: an armamentarium in future airway management
Introduction
Point-of-care ultrasound (POCUS) has the advantage in its simplistic, non-invasive and portable nature enabling rapid assessment without depending on transfers to the radiology department. The integration of upper airway ultrasound into POCUS examination paved the way to a paradigm shift in upper airway assessment (1). This article addresses the role of upper airway ultrasound as a potential first line airway assessment tool.
Sonoanatomy of focused the upper airway ultrasound
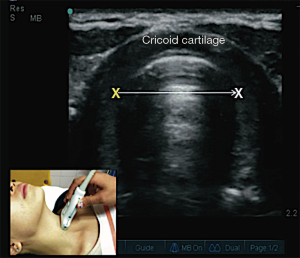
Understanding the physics of basic ultrasound and being proficient in the upper airway anatomy will enhance mastery of sonoanatomy. Tracheal cartilage in the transverse plane is viewed as an inverted ‘U’ shaped structure (Figure 1), bordered posteriorly by a hyperechoic undeviating strip line, the air-mucosal (A-M) interface. Cricoid cartilage is best seen when the probe moves cephalad (Figure 2). This is shown sonographically as a C-shaped mixed-echoic structure, much thicker than the tracheal cartilages, with a similar hyperechoic strip line of A-M interface directly beneath it. The cricothyroid membrane is seen on the transverse view as a hyperechoic strip line (Figure 3) sandwiched between the cricoid and thyroid cartilage. Transverse view at the level of the thyroid cartilage provides the best window to visualize the vocal cords. Hyperechoic vocal ligaments on the medial aspect delineate the vocal cords (2). The thyroid cartilage appears as a hypoechoic triangular structure in this view (Figure 4)
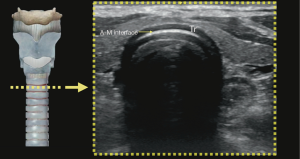
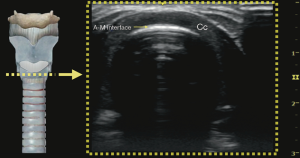
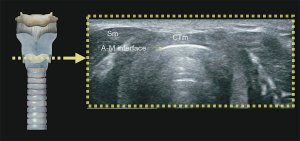
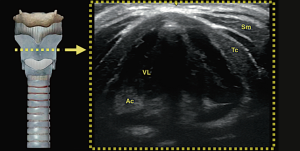
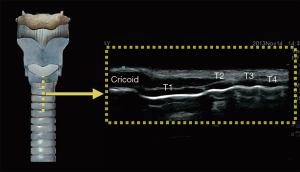
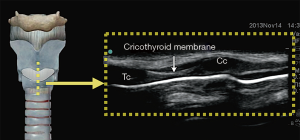
In the longitudinal plane, the tracheal cartilages (T1, T2, T3, T4) have a hypoechoic appearance with a similar A-M interface beneath them (Figure 5). Cricoid cartilage is the bulkiest cartilage cephalad to the tracheal rings. The tracheal cartilage sequence is also knowns as “string of beads”. A linear hyperechoic line seen posteriorly on longitudinal plane of upper airway is formed by reverberation artefacts known as the A-M interface (1). The cricothyroid membrane in the longitudinal view (Figure 6) is a membrane near the A-M interface in between the cricoid and thyroid cartilages.
Clinical applications of focused upper airway ultrasound
Upper airway ultrasound can be used for the evaluation of:
- Airway size and prediction of
- Endotracheal tube (ETT) size;
- Left double-lumen bronchial tube size.
- Prediction of difficult laryngoscopy;
- Airway device placement and depth:
- ETT confirmation;
- ETT depth;
- Laryngeal mask airway (LMA) confirmation.
- Procedures:
- Percutaneous cricothyroidotomy;
- Percutaneous dilatational tracheostomy (PDT);
- Superior laryngeal nerve blocks for awake fiberoptic intubation.
- Identifying pathological airway structures:
- Epiglottis;
- Vocal cord assessment;
- Trachea location and surrounding structures;
- Laryngeal injury.
- Predicting post extubation stridor.
Airway size and prediction of ETT and left double-lumen bronchial tube size
The accuracy of ultrasound evaluation of airway size is validated against magnetic resonance imaging (2) and computed tomography scan (3). A correctly sized ETT can potentially avoid the hazards of subglottic stenosis and inadequate ventilation especially in the paediatric population, where the narrowest airway lies in the subglottic region. Subglottic airway diameter can be measured ultrasonographically (Figure 7).
Ultrasound measurement of the subglottic diameter is superior to age-based (4-6) and height-based formula (4) in estimating suitability of ETT size (Figure 7). It has the ability to predict accurately up to 98% of cuffed ETT size and 96% of uncuffed ETT size. Age and height-based formula, in comparison only managed to predict 35% of cuffed and 60% of uncuffed ETT size, according to a study by Shibasaki et al., Kim et al. based on ultrasonographic measurements of the airway proposed a formula to choose the appropriate ETT size in children (7).
Selection of a left-sided double lumen tube can be based on ultrasound measurement of the outer tracheal width at the level just above the sternoclavicular junction. This measurement has been shown to correlate with the internal tracheal width and left mainstem bronchus size on the computed tomography scan (3).
Prediction of difficult laryngoscopy
The ability to predict a difficult airway can potentially save lives and remained an area of great research interests across various disciplines. Although most were pilot studies with small study samples, preliminary findings showed promising results. There were four studied methods to date:
(I) Visualisation of hyoid bone
The inability to visualise the hyoid bone on ultrasound using the sublingual approach predicts difficult intubation as demonstrated by Hui et al. This method has high sensitivity and specificity with a high positive likelihood ratio of 21.6 and moderate negative likelihood ratio of 0.28 (8).
(II) Hyomental distance ratio
Wojtczak et al. demonstrated that a shorter hyomental distance ratio of 1–1.05 in morbidly obese patients predicts difficult laryngoscopy with high sensitivity. This is the distance between hyoid bone and mandibular mentum in the neutral position to the hyperextended neck position. Patients intubated easily were found to have a hyomental distance ratio ranging 1.12–1.16 (9).
(III) Anterior neck thickness
Anterior neck thickness at different anatomical levels has been found to be predictors for difficult intubation (10-13). These are studied at the level of the vocal cords, hyoid bone and thyrohyoid membrane. At the level of the vocal cords, Ezri et al. found that a mean pretracheal tissue exceeding 28±2.7 mm in the obese patients increases the risk of difficult laryngoscopy (10). This was not reproducible in different population (11-13). Adhikari et al. however found that, anterior neck thickness above 2.8cm at the level of the hyoid bone and thyrohyoid membrane better predicts difficult laryngoscopy compared to that of the vocal cords (12). Pinto et al. supported the latter findings at the level of thyrohyoid membrane (13).
(IV) Tongue thickness and tongue thickness to thyromental distance ratio
Tongue thickness of more than 6.1 cm measured using the submental approach; and higher tongue thickness to thyromental distance ratio of more than 0.87 are capable of predicting difficult tracheal intubation (14).
More consistent results with higher sample size providing level one evidence are imperative to mandate its use for routine screening pre-intubation.
ETT confirmation
Capnography has traditionally been regarded as the gold standard for ETT placement confirmation. This is not without limitations. Cardiorespiratory arrest (15), low flow states, bronchoconstriction and technical malfunction or availability (16-18), for instance, may preclude its use. In the emergency situation, ultrasonography may be more reliable and accessible (19).
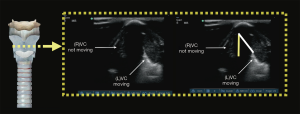
Adi et al. demonstrated that upper airway ultrasound is as good as waveform capnography for ETT confirmation, with a kappa value of 0.85 showing good agreement between these two methods. There was no delay in confirmation using ultrasound, with short mean time of 16.4±7.3 seconds, which increases practicality of this method. On the ultrasound, the ETT is seen as “double tract sign” (16) (Figure 8).
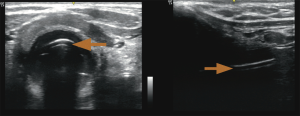
Tracheal rapid ultrasound exam (T.R.U.E.), a method by Chou et al. using static transtracheal approach at the level of the suprasternal notch, can diagnose oesophageal intubation with high sensitivity (98.9%) and specificity (94.1%) (20).
ETT depth
Clinical assessment by auscultation and observing chest rise may fail to identify up to 55% of endobronchial intubations (21). The avoidance of endobronchial intubation could be accomplished with the aid of ultrasonography.
Tracheal rapid ultrasound saline test (TRUST), a technique using saline-filled ETT cuff by Tessaro et al., showed accurate and rapid assessment of depth of ETT placement to prevent endobronchial intubation in children (22). Even novice sonographers could accurately identify a saline-inflated ETT cuff at the level of the suprasternal notch in a cadaver study by Uya et al. (23).
LMA confirmation
Ultrasound can be used to detect LMA malrotation in children with high sensitivity and specificity (93% and 82% respectively) and an accuracy of 87% (24). Kim et al. in this observational study in paediatric patients correlated upper airway ultrasound with fiberoptic bronchoscopy findings, and recognised LMA malrotation based on graded sonographic arytenoid cartilage elevation in the transverse plane (24).
Sonographic visualisation of the LMA cuff is also possible when cuffs are inflated with saline and contrast agents (25).
Percutaneous cricothyroidotomy
Cricothyroidotomy is a life-saving procedure in the “cannot intubate cannot ventilate” situation. Ultrasound can be used both pre-procedural by surface marking the cricothyroid membrane prior to an anticipated difficult intubation, or as a real-time procedural guidance.
Upper airway ultrasound improves procedural safety by providing accurate landmark particularly when anatomy is not easily identified by traditional palpation method (26-28). Siddiqui et al. in a randomised trial on cadavers compared safety of cricothyroidotomy between two groups, cricothyroid membrane landmark by ultrasound and by digital palpation. Airway injuries were three times lower when cricothyroid membrane is identified using ultrasound guidance than by digital palpation even in those with distorted neck anatomy, although time to completion of procedure almost doubled (29)
Locating the cricothyroid membrane is fast with short learning curve (30); and cricothyroidotomy can be performed successfully in a quick manner (31), an important feature in the emergency situation. Nicholls et al found that it took a mean of 24±20 seconds to identify the cricothyroid membrane in the emergency department (29). Curtis et al. proved that real-time ultrasound-guided, bougie- assisted cricothyroidotomy in cadavers is feasible with high success rate. The median time to identify the cricothyroid membrane was less than 4 seconds in this study, and to completion of procedure, 26 seconds (31).
PDT
Upper airway ultrasound improves safety of PDT (32-34). It enables precise location of procedure site (35), allows selection of tracheostomy tube size and length (36) and avoid trauma to the airway (37), vessels and anterior neck structures (32,33). Twenty-five percent of patients underwent re-siting of puncture site after ultrasound assessment (33).
Both pre-procedural and real-time ultrasound guidance is beneficial especially in patients with distorted anatomy. The feasibility of real-time in-plane ultrasound guided PDT was not restricted only to cadavers (38) but also in real life situations.
In the critically ill patients, the success and complication rates of ultrasound guided PDT in the TRACHUS randomised controlled trial is similar to that of bronchoscopy-guided PDT, a tool considered standard for PDT (39).
Ultrasound guided PDT is also superior to anatomical landmark method in the following studies. Dinsmore et al. found that ultrasound guided PDT is superior with higher successful cannulation rate in shorter time (40). This advantage is also illustrated in the Traditional landmArk versus ultRasound Guided Evaluation Trial (TARGET) study (41) and by Dinh et al. (42), who demonstrated less attempts to successful cannulation, with high puncture accuracy.
Ultrasound-guided translaryngeal blocks
Real time sonographic guided superior laryngeal nerve blocks is useful to facilitate awake fibreoptic intubation under direct visualisation of the nerve (43).
Evaluation of pathological airway structures
Evaluation of epiglottis
The normality of the epiglottis can be evaluated in terms of thickness and shape.
Patients diagnosed clinically with epiglottitis gave the appearance of a thickened epiglottis on the ultrasound (44). There is however no standard cut-off limit for epiglottic thickness to date, although preliminary studies have evaluated epiglottis thickness in healthy population (45)
Hung et al. described the appearance of the “alphabet P sign” in patients with epiglottitis, the result of a hyperechoic thickened epiglottis in relation to the acoustic shadow of the hyoid bone with the transducer placed longitudinally at the level of the thyrohyoid membrane (46).
Further research is required to determine the average size of the epiglottis in the healthy population.
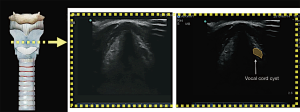
Vocal cord assessment
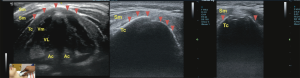
Real-time visualisation of vocal cords movement during quiet breathing and upon vocalisation enable detection of vocal cord palsy (Figure 9) and vocal cord pathology non-invasively (Figure 10). The adduction and abduction of vocal cords can be seen clearly on ultrasound.
Trachea location and surrounding structures
The image showed the presence of a large goitre causing tracheal displacement to the left (Figure 11).
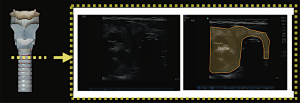
Laryngeal injury
Blunt neck trauma can result in life-threatening upper airway injuries leading to airway obstruction. POCUS allows identification of deviation from the norm, offering an opportunity to detect laryngeal injuries by the bedside according to Schaefer classification (Figure 12). This can expedite emergency management especially in those deemed unstable for transport to the imaging department.
Predicting postextubation stridor and airway oedema
Ultrasound measurement of air column width difference at the level of the vocal cords before and after ETT cuff deflation has the potential to predict postextubation stridor. This difference represents the amount of air passing through the vocal cords. A smaller difference is an indirect indication of narrower airway and possible laryngeal oedema, which may present clinically as postextubation stridor (47,48).
Sutherasan et al. proposed that air column width difference of less than 1.6 mm be taken to predict postextubation stridor. The sensitivity and specificity of this value was both 70% with a high negative predictive value and low positive predictive value (92% and 32% respectively) (48). This was however not reproducible by Mikaeili et al. (49).
The cutoff value to predict postextubation stridor may differ in different population. The use of ultrasound to predict postextubation stridor will require further studies with bigger sample size in different population.
Upper airway ultrasound educational learning curve
The quality and results of upper airway ultrasound demand not only technical proficiency, but also competent interpretation of sonogram images. It is operator-dependent, necessitating adequate training and experience. Fortunately, studies showed shallow educational learning curves, and skills can be mastered easily for interested parties with little initial ultrasound knowledge (22,50,51).
There are several reasons contributing to the relatively short learning curve. Unlike other emergency ultrasound applications, the location of the upper airway is fixed, and do not require much adjustments once location is identified. The anatomy is consistent among patients even in the obese, easy to identify with less dependence on body habitus, unlike abdominal scans (51).
Gottlieb et al. showed that the four-step (4S) technique to confirm ETT placement in the adult human cadaver model provide a basic learning platform for both experts and novices. Both are able to confirm placement of ETT with a mean time of less than 30 seconds although novices took one and a half times longer with less accuracy in the obese cadaver. Otherwise, this learning technique provides high sensitivity and specificity in the detection of proper ETT placement (51). In another study on emergency medicine fellows with limited baseline ultrasound knowledge, a 50-minute combined theoretical and practical training session can identify tracheal location and ETT depth with high sensitivity (23).
Chenkin et al. again supported the ease of acquiring upper airway ultrasound knowledge in his study. Emergency physicians were able to interpret accurately esophageal and endotracheal intubation ultrasound clips with 100% success rate after only a brief online 10-minute tutorial and two practice attempts (50).
Conclusions
Upper airway ultrasound is a convenient, cost-effective and reproducible tool. The integration of upper airway ultrasound to complement repertoire of pre-intubation airway screening may be the way forward in the future standard of care. It is a potential first-line airway assessment and management tool.
Acknowledgments
We would like to thank Dr. Lai Si Qi, World Integrated Network for Focused Ultrasound (WINFOCUS) Malaysia, and Ipoh Emergency Medicine Society (IEMS) for their assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Osman A, Sum KM. Role of upper airway ultrasound in airway management. J Intensive Care 2016;4:52. [Crossref] [PubMed]
- Lakhal K, Delplace X, Cottier JP, et al. The feasibility of ultrasound to assess subglottic diameter. Anesth Analg 2007;104:611-4. [Crossref] [PubMed]
- Sustić A, Miletić D, Protić A, et al. Can ultrasound be useful for predicting the size of a left double-lumen bronchial tube? Tracheal width as measured by ultrasonography versus computed tomography. J Clin Anesth 2008;20:247-52. [Crossref] [PubMed]
- Shibasaki M, Nakajima Y, Ishii S, et al. Prediction of Pediatric Endotracheal Tube Size by Ultrasonography. Anesthesiology 2010;113:819-24. [Crossref] [PubMed]
- Bae JY, Byon HJ, Han SS, et al. Usefulness of ultrasound for selecting a correctly sized uncuffed tracheal tube for paediatric patients. Anaesthesia 2011;66:994-8. [Crossref] [PubMed]
- Schramm C, Knop J, Jensen K, et al. Role of ultrasound compared to age-related formulas for uncuffed endotracheal intubation in a pediatric population. Paediatr Anaesth 2012;22:781-6. [Crossref] [PubMed]
- Kim EJ, Kim SY, Kim WO, et al. Ultrasound measurement of subglottic diameter and an empirical formula for proper endotracheal tube fitting in children. Acta Anaesthesiol Scand 2013;57:1124-30. [Crossref] [PubMed]
- Hui CM, Tsui BC. Sublingual ultrasound as an assessment method for predicting difficult intubation: A pilot study. Anaesthesia 2014;69:314-9. [Crossref] [PubMed]
- Wojtczak JA. Submandibular sonography: assessment of hyomental distances and ratio, tongue size, and floor of the mouth musculature using portable sonography. J Ultrasound Med 2012;31:523-8. [Crossref] [PubMed]
- Ezri T, Gewürtz G, Sessler DI, et al. Prediction of difficult laryngoscopy in obese patients by ultrasound quantification of anterior neck soft tissue. Anaesthesia 2003;58:1111-4. [Crossref] [PubMed]
- Komatsu R, Sengupta P, Wadhwa A, et al. Ultrasound quantification of anterior soft tissue thickness fails to predict difficult laryngoscopy in obese patients. Anaesth Intensive Care 2007;35:32-7. [Crossref] [PubMed]
- Adhikari S, Zeger W, Schmier C, et al. Pilot study to determine the utility of point-of-care ultrasound in the assessment of difficult laryngoscopy. Acad Emerg Med 2011;18:754-8. [Crossref] [PubMed]
- Pinto J, Cordeiro L, Pereira C, et al. Predicting difficult laryngoscopy using ultrasound measurement of distance from skin to epiglottis. J Crit Care 2016;33:26-31. [Crossref] [PubMed]
- Yao W, Wang B. Can tongue thickness measured by ultrasonography predict difficult tracheal intubation? Br J Anaesth 2017;118:601-9. [Crossref] [PubMed]
- Chou HC, Chong KM, Sim SS, et al. Real-time tracheal ultrasonography for confirmation of endotracheal tube placement during cardiopulmonary resuscitation. Resuscitation 2013;84:1708-12. [Crossref] [PubMed]
- Adi O, Chuan TW, Rishya M. A feasibility study on bedside upper airway ultrasonography compared to waveform capnography for verifying endotracheal tube location after intubation. Crit Ultrasound J 2013;5:7. [Crossref] [PubMed]
- Takeda T, Tanigawa K, Tanaka H, et al. The assessment of three methods to verify tracheal tube placement in the emergency setting. Resuscitation 2003;56:153-7. [Crossref] [PubMed]
- MacLeod BA, Heller MB, Gerard J, et al. Verification of endotracheal tube placement with colorimetric end-tidal CO2 detection. Ann Emerg Med 1991;20:267-70. [Crossref] [PubMed]
- Das SK, Choupoo NS, Haldar R, et al. Transtracheal ultrasound for verification of endotracheal tube placement: a systematic review and meta-analysis. Can J Anaesth 2015;62:413-23. [Crossref] [PubMed]
- Chou HC, Tseng WP, Wang CH, et al. Tracheal rapid ultrasound exam (T.R.U.E.) for confirming endotracheal tube placement during emergency intubation. Resuscitation 2011;82:1279-84. [Crossref] [PubMed]
- Sitzwohl C, Langheinrich A, Schober A, et al. Endobronchial intubation detected by insertion depth of endotracheal tube, bilateral auscultation, or observation of chest movements: Randomised trial. BMJ 2010;341:c5943. [Crossref] [PubMed]
- Tessaro MO, Salant EP, Arroyo AC, et al. Tracheal rapid ultrasound saline test (T.R.U.S.T.) for confirming correct endotracheal tube depth in children. Resuscitation 2015;89:8-12. [Crossref] [PubMed]
- Uya A, Spear D, Patel K, et al. Can novice sonographers accurately locate an endotracheal tube with a saline-filled cuff in a cadaver model? A pilot study. Acad Emerg Med 2012;19:361-4. [Crossref] [PubMed]
- Kim J, Kim JY, Kim WO, et al. An Ultrasound Evaluation of Laryngeal Mask Airway Position in Pediatric Patients: An Observational Study. Anesth Analg 2015;120:427-32. [Crossref] [PubMed]
- Wojtczak JA, Cattano D. Laryngo-tracheal ultrasonography to confirm correct endotracheal tube and laryngeal mask airway placement. J Ultrason 2014;14:362-6. [Crossref] [PubMed]
- Aslani A, Ng SC, Hurley M, et al. Accuracy of identification of the cricothyroid membrane in female subjects using palpation: An observational study. Anesth Analg 2012;114:987-92. [Crossref] [PubMed]
- Bair AE, Chima R. The Inaccuracy of Using Landmark Techniques for Cricothyroid Membrane Identification: A Comparison of Three Techniques. Acad Emerg Med 2015;22:908-14. [Crossref] [PubMed]
- Elliott DS, Baker PA, Scott MR, et al. Accuracy of surface landmark identification for cannula cricothyroidotomy. Anaesthesia 2010;65:889-94. [Crossref] [PubMed]
- Siddiqui N, Arzola C, Friedman Z, et al. Ultrasound Improves Cricothyrotomy Success in Cadavers with Poorly Defined Neck Anatomy. Anesthesiology 2015;123:1033-41. [Crossref] [PubMed]
- Nicholls SE, Sweeney TW, Ferre RM, et al. Bedside sonography by emergency physicians for the rapid identification of landmarks relevant to cricothyrotomy. Am J Emerg Med 2008;26:852-6. [Crossref] [PubMed]
- Curtis K, Ahern M, Dawson M, et al. Ultrasound-guided, bougie-assisted cricothyroidotomy: A description of a novel technique in cadaveric models. Acad Emerg Med 2012;19:876-9. [Crossref] [PubMed]
- Hatfield A, Bodenham A. Portable ultrasonic scanning of the anterior neck before percutaneous dilatational tracheostomy. Anaesthesia 1999;54:660-3. [Crossref] [PubMed]
- Kollig E, Heydenreich U, Roetman B, et al. Ultrasound and bronchoscopic controlled percutaneous tracheostomy on trauma ICU. Injury 2000;31:663-8. [Crossref] [PubMed]
- Guinot PG, Zogheib E, Petiot S, et al. Ultrasound-guided percutaneous tracheostomy in critically ill obese patients. Crit Care 2012;16:R40. [Crossref] [PubMed]
- Sustić A, Kovac D, Zgaljardić Z, et al. Ultrasound-guided percutaneous dilatational tracheostomy: a safe method to avoid cranial misplacement of the tracheostomy tube. Intensive Care Med 2000;26:1379-81. [Crossref] [PubMed]
- Rajajee V, Fletcher JJ, Rochlen LR, et al. Real-time ultrasound-guided percutaneous dilatational tracheostomy: a feasibility study. Crit Care 2011;15:R67. [Crossref] [PubMed]
- Rajajee V, Williamson CA, West BT. Impact of real-time ultrasound guidance on complications of percutaneous dilatational tracheostomy: A propensity score analysis. Crit Care 2015;19:198. [Crossref] [PubMed]
- Kleine-Brueggeney M, Greif R, Ross S, et al. Ultrasound-guided percutaneous tracheal puncture: a computer-tomographic controlled study in cadavers. Br J Anaesth 2011;106:738-42. [Crossref] [PubMed]
- Gobatto ALN, Besen BAMP, Tierno PFGMM, et al. Ultrasound-guided percutaneous dilational tracheostomy versus bronchoscopy-guided percutaneous dilational tracheostomy in critically ill patients (TRACHUS): a randomized noninferiority controlled trial. Intensive Care Med 2016;42:342-51. [Crossref] [PubMed]
- Dinsmore J, Heard AM, Green RJ. The use of ultrasound to guide time-critical cannula tracheotomy when anterior neck airway anatomy is unidentifiable. Eur J Anaesthesiol 2011;28:506-10. [Crossref] [PubMed]
- Rudas M, Seppelt I, Herkes R, et al. Traditional landmark versus ultrasound guided tracheal puncture during percutaneous dilatational tracheostomy in adult intensive care patients: a randomised controlled trial. Crit Care 2014;18:514. [Crossref] [PubMed]
- Dinh VA, Farshidpanah S, Lu S, et al. Real-time Sonographically Guided Percutaneous Dilatational Tracheostomy Using a Long-Axis Approach Compared to the Landmark Technique. J Ultrasound Med 2014;33:1407-15. [Crossref] [PubMed]
- Sawka A, Tang R, Vaghadia H. Sonographically guided superior laryngeal nerve block during awake fiberoptic intubation. A A Case Rep 2015;4:107-10. [Crossref] [PubMed]
- Ko DR, Chung YE, Park I, et al. Use of Bedside Sonography for Diagnosing Acute Epiglottitis in the Emergency Department. J Ultrasound Med 2012;31:19-22. [Crossref] [PubMed]
- Werner SL, Jones RA, Emerman CL. Sonographic Assessment of the Epiglottis Sandra. Acad Emerg Med 2004;11:1358-60. [Crossref] [PubMed]
- Hung TY, Li S, Chen PS, et al. Bedside ultrasonography as a safe and effective tool to diagnose acute epiglottitis. Am J Emerg Med 2011;29:359.e1-3. [Crossref] [PubMed]
- Ding LW, Wang HC, Wu HD, et al. Laryngeal ultrasound: A useful method in predicting post-extubation stridor. A pilot study. Eur Respir J 2006;27:384-9. [Crossref] [PubMed]
- Sutherasan Y, Theerawit P, Hongphanut T, et al. Predicting laryngeal edema in intubated patients by portable intensive care unit ultrasound. J Crit Care 2013;28:675-80. [Crossref] [PubMed]
- Mikaeili H, Yazdchi M, Tarzamni MK, et al. Laryngeal Ultrasonography Versus Cuff Leak Test in Predicting Postextubation Stridor. J Cardiovasc Thorac Res 2014;6:25-8. [PubMed]
- Chenkin J, McCartney CJ, Jelic T, et al. Defining the learning curve of point-of-care ultrasound for confirming endotracheal tube placement by emergency physicians. Crit Ultrasound J 2015;7:14. [Crossref] [PubMed]
- Gottlieb M, Bailitz JM, Christian E, et al. Accuracy of a Novel Ultrasound Technique for Confirmation of Endotracheal Intubation by Expert and Novice Emergency Physicians. West J Emerg Med 2014;15:834-9. [Crossref] [PubMed]
Cite this article as: Adi O, Kok MS, Abdull Wahab SF. Focused airway ultrasound: an armamentarium in future airway management. J Emerg Crit Care Med 2019;3:31.