
Perioperative goal directed therapy—current view
Introduction
Cardiovascular (CV) morbidity and mortality is one of the major health-care problems. Almost 18 million patients die annually related to CV disease (1). Though our ability to treat CV diseases has improved significantly over the last decades, the prevalence of patients with decreased CV reserve is increasing (1). Besides, 313 million patients undergo surgery annually all over the world and 4.2 million presumably die within 30 days after surgery (2). It has been demonstrated previously, that these deaths frequently occur in small proportion (circa 10%) of high-risk patients (often with CV limitations) (3,4). These patients also consume much higher proportion of the global budget of perioperative care (5). Costs attributed to social care associated with decreased quality of life remain unresolved, but are presumably even higher.
The major driver of unfavorable postoperative outcome seems to be the patient’s low functional reserve (6). The perioperative goal directed therapy (pGDT) was designed to optimize patients’ CV performance and thus lower the risk of major complications in the perioperative period. Currently, this approach encompasses number of possible targets and/or treatment algorithms, which has been associated with decreased postoperative complications and improved outcome based on several large meta-analyses (7-11).
Historical perspective and physiological rationale
In 1988, Shoemaker et al. published seminal paper in which they described the concept of oxygen debt and its relevance for postsurgical period and development of complications (12). According to these data, surgical trauma and following period of healing are coupled with increased tissue oxygen consumption. This normally leads to increase of cardiac output and modulation of systemic vascular resistance in order to increase tissue oxygen supply. Patients able to cope with these increased demands usually pass the perioperative period without organ failure (13). Median of cardiac index (CI) observed by Shoemaker et al. was 4.5 L/min/m2 (coupled with oxygen delivery of 600 mL/min/m2 and oxygen consumption of 170 mL/min/m2). In next prospective interventional study, Shoemaker et al. have demonstrated that iatrogenic increase of hemodynamic parameters to reach these predefined goals improved postoperative outcome in high risk surgical patients (12). In following decades, several other studies have evaluated this approach in various settings (14-18). Trials aimed on hemodynamic optimization of patients in intensive care have systematically failed to demonstrate any benefit (16-18). The reason for this has been attributed to the fact that dead tissue does not need the oxygen (19). The preemptive use in high-risk surgical patients seems to be the cornerstone of pGDT.
To understand the concept of pGDT one has to study the tissue perfusion physiology in detail. Because no stores of oxygen are available at the cellular level, the tissue is supplied on a continuous basis. In order to keep the adequate oxygen supply to tissues either its content in blood or the blood flow has to be modulated or organ demands has to be lowered. However, any change on global (macrocirculatory) level does not necessarily translate into the local/tissue (microcirculatory) level.
Blood supply to various organs is autoregulated to keep the constant blood flow under wide range of blood pressure, but outside these borders the local flow is dependent on systemic blood pressure. Hence, severe systemic hypotension or global hypoperfusion lead also to tissue hypoperfusion. Even more important is the redistribution of blood flow to “vital” organs (i.e., heart and brain). To maintain flow in vital organs the body decreases flow to “less important” tissues in case of severe hypoperfusion—therefore gastrointestinal tract, kidneys, skin, etc. may suffer undetected malperfusion (so called occult hypoperfusion). In 2004, Meregalli et al. have demonstrated, that signs of occult hypoperfusion (in this case increased serum lactate level) in spite of normal macrocirculatory parameters (normal blood pressure, etc.) were associated with unfavorable postsurgical outcome (20).
However, not all the determinants of the oxygen delivery equation (Figure 1) are clinically equal. If we presume a clinical case of a patient after surgical procedure having an oxygen delivery index of 450 mL/min/m2, in whom we would like to increase it to 600 mL/min/m2 (Figure 2). A change in CI of 33% (means absolute increase of 1 L/min/m2) is usually affordable without problems. In contrary, the equal increase caused by elevation of the hemoglobin concentration would be hardly clinically acceptable, because the only way to modulate hemoglobin concentration perioperatively is coupled with transfusion of allogenic blood. Thus, the benefit of hemoglobin increase is largely limited by the risks of transfusion related complications. Moreover, according to current standards and guidelines the hemoglobin level ranging 7.0–1.0 g/L is taken as a sufficient (21).
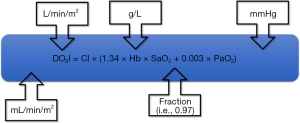
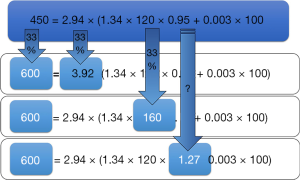
Because of normally high oxygen saturation, desired increase is not affordable by oxygen therapy. An increase in oxygen saturation from 95% to 100% would be a mere 5% increase of oxygen delivery without further possibility to increase it under normobaric conditions. Therefore, modulating CI remains the cornerstone of pGDT. As previously stated in the concept of the functional hemodynamic monitoring (22), this is possible by modulating any of the three parameters affecting stroke volume (SV)—preload, contractility or afterload. This approach usually works quite well, but still there are some limitations. For instance, increasing the preload using fluids works only in patients operating on the steep portion of Frank-Starling curve, which is the majority of surgical patients. Inotropes (dobutamine or dopexamine) do increase heart contractility (and/or chronotropy), but for a price of increased myocardial oxygen consumption. Finally, afterload is usually modulated to reach adequate perfusion pressures, but this may be on the price of regional flow redistribution. Moreover, improving macrocirculatory variables need not directly link to improved microcirculation (so called micro-macro incoherence) (23). Disturbances in the microcirculation (capillary density, perfusion heterogeneity, etc.) may significantly impair the availability of substrates at the cellular level. In fully developed shock states (for instance in sepsis) this phenomenon may limit tissue perfusion and cellular metabolism even under normal systemic circulatory conditions. Red cell transfusion may impair microcirculation and blood rheology and fluids may contribute to edema formation and prolongation of diffusion distance.
Summary of available evidence
Over the last four decades starting with Shoemaker the concept of pGDT evolved in terms of monitoring devices used, populations studied and treatment goals reached. Several meta-analyses on this subject has been published over the last 10 years (7-9,11,24). In the two most recent ones the authors were able to identify 95 (7) and 112 (8) randomized controlled studies. Based on the meta-analysis by Chong et al. (7), the pGDT was associated with decreased mortality, morbidity and hospital length of stay (see Table 1). A major problem of such meta-analytical work is the great heterogeneity among included trials inducing a risk of bias. Therefore, authors of the last review (8) decided not to perform any kind of meta-analysis.

Full table
However, even these heterogeneous results may allow us to draw some conclusions. Based on our long-term screening of literature supported by results of multiple databases searches [i.e., for the purpose of previous meta-analysis (9)] our group is aware of 118 pGDT randomized prospective studies in human subjects published on this topic so far (as for May 2019) (Table 2).
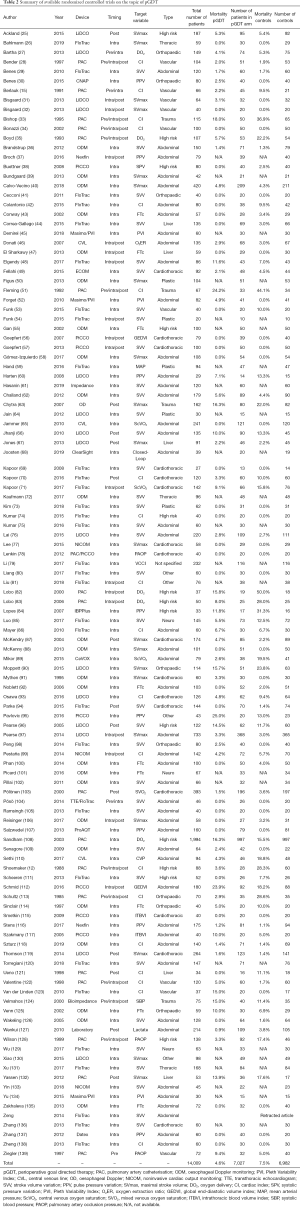
Full table
Evolution of monitoring technologies
Naturally, the story began with pulmonary artery catheterisation (PAC): altogether 19 randomized controlled trials (RCTs) (accounting for 3,706 patients) have been published on pGDT driven by PAC so far [the study by Yassen in 2012 (132) is currently the last one]. PAC has many disadvantages for the pGDT protocols—first, it is highly invasive with high risk of complications. Besides, the pulmonary artery occlusion pressure (PAOP) is not a reliable predictor of fluid responsiveness (140). Contrary, PAC has always been recognized as the gold standard of CI monitoring. Nevertheless, based on our current knowledge the PAC is nowadays replaceable by less invasive devices and will have probably only minor impact on the future pGDT.
Transpulmonary dilution devices (PiCCO, VolumeView, LiDCO Plus) replaced the PAC monitoring because of their acceptable reliability and much easier applicability in many indications (especially for the critically ill). However, for the pGDT these devices never played a major role: mostly because the need of cannulation of central vein and major artery (mostly femoral), time-consuming calibration and costs. Only seven studies have been published using transpulmonary dilution techniques, and it seems the volumetric indices (unique for this kind of monitoring) bring no more additional value.
Oesophageal Doppler technology (OED) is probably the second most important pGDT monitoring device. It was the first that replace the PAC in many countries, and the pGDT is virtually associated with OED. The National Institute for Health and Care Excellence (NICE) guidelines (141) has implemented OED monitoring into the national-wide healthcare program. Up to day, 25 studies have been published using the OED technology, in which 2,709 patients were included. Its use is less invasive and has minimum contraindications, though sometimes it could be difficult to obtain the good acoustic window and performance of the device may be disturbed by the ongoing surgery. The CI/SV measurement is relatively accurate (142) and offers specific parameters of fluid response [corrected flow time (FTc)] and contractility (mean acceleration or peak velocity). Hence, the OED enables parallel assessment of individual heart performance determinants—as demonstrated by Szturz recently (118).
Devices based on pulse wave analysis (PWA) represent the largest and most frequently used group. They have been used in 51 RCTs so far and they are base for several ongoing multicentric RCTs (143,144) (plus the @OPTIMISE2trial). The simplicity to use (virtually “plug-and-play”) make them ideal for pGDT in intermediate to high-risk surgical patients. Most of uncalibrated PWA technologies have been repeatedly questioned in terms of the reliability (accuracy and precision); but it seems the tracking ability is good enough to enable pGDT (145,146). Besides, the use of PWA is mostly coupled with the use of dynamic variations of pulse pressure or SV as markers of preload responsiveness.
Non-invasive devices are currently the most controversial group of monitors. First, the group is extremely heterogeneous and the devices are based on different principles (volume clamp method of blood pressure curve reconstruction, thoracic bioimpedance or bioreactance, Fick principle and multiple others). This complete non-invasiveness virtually enables widespread use, even for patients in low-to-intermediate risk, because they are not associated with any potential harm. However, accuracy and precision of these devices has been repeatedly questioned (146,147), even though the population tested is not entirely matching the target population [mostly high-risk or intensive care patients—for further reading see (148)]. Among screened studies 13 RCTs used some sort of non-invasive technology (10 out of them has been published during or after 2015). In four RCTs the non-invasive blood pressure analysis with following non-calibrated cardiac output calculation has been used (hence are more an extension of PWA devices). In three other studies, Massimo rainbow pulse oximeter and its Pleth Variability Index (PVI) parameter has been used hence only fluid based pGDT was affordable. Finally, bioimpedance or bioreactance technology has been used in six another RCTs.
Treatment goals and means
Cardiac output/index is historically the most important and reasonable treatment target of pGDT protocols. Coupled with hemoglobin concentration it creates the physiological rationale for improving the peripheral tissue oxygen supply. However, the actual value of CI target significantly varies among studies. The original work of Shoemaker and colleagues (12) aimed for “supranormal” CI of 4.5 L/min/m2—goal not easily to reach in high-risk patients without using inotropic support. In later RCTs only normal values (i.e., around 3.0 L/min/m2) were used as target and most recently concept of “avoid-the-low CI” was used (i.e., above 2.0 or 2.5 L/min/m2). Patients in the intermediate-risk group usually need only preload optimization to reach these conservative targets. Therefore, further inotropic support is necessary only in minority of patients and should be limited for high-risk patients or those with unexpectedly low cardiac performance. Individualization of the target CI base on preoperative echocardiography or by other non-invasive measurements may be and interesting option for the future.
SV/SV index as a treatment target is mostly used to improve the heart preload. The NICE/National Health Service (NHS) guidelines-based protocol (149) recommend a stepwise SV maximization process: a step/volume challenge of 200–250 mL is given with reassessment. At least 10% increase in SV after volume challenge is taken as a positive response that prompts a repetition of volume challenge. The goal is to reach a state of fluid unresponsiveness by sequential volume loading steps. If any decrease in SV larger than 10% occurs later on, a volume challenge should be repeated to keep maximal SV conditions. Interestingly, the other factors affecting the SV (contractility or afterload changes) are frequently neglected. In addition, in patients with good CV reserve, the maximization of SV seems not to be associated with improved outcomes (62). It seems that reaching fluid unresponsiveness by SV maximization may lead to unnecessarily high amount of intravenous fluids administration with all its negative consequences (especially considering the vasodilatory effect of anesthetics).
Variation of SV, pulse pressure or plethysmography-variability index create together one large group of so-called “dynamic preload parameters”—parameter yet unbeaten in terms of predictive potential for testing preload reserve under generally known and acceptable conditions (i.e., absence of spontaneous breathing, tidal volume of more than 8 mL/kg of ideal body weight, absence of arrhythmias, etc.) (150-152). Fluid optimization guided by these parameters has been associated with improved outcomes based on meta-analysis of 14 studies (9). Naturally, these parameters may be used for optimizing the fluid load only, thus they have to be coupled with parameters assessing oxygen delivery adequacy and afterload.
Among Doppler-based parameters, the FTc has gained most attention as a parameter for guiding preload optimization. Other parameters (SV, CI and/or peak velocity) are usually used for complex assessment of hemodynamic status and further optimization. Low specificity of FTc and its dependence on afterload and some demographic parameters (i.e., age, sex) are drawbacks of this approach. Contrary, peak velocity is currently the most appropriate parameter to assess the contractile function. In a recent RCT by Szturz such complex approach with the use of FTc to asses fluid needs, peak velocity to modulate contractility by dobutamine and blood pressure product (systemic resistance) to use vasoactive medication has been associated with improved outcomes in abdominal surgery (118).
Blood pressure is often overlooked target of the pGDT protocols. Adequate perfusion of vital organs is maintained by body regulatory mechanism throughout large scale of perfusion pressures via its natural autoregulatory mechanisms. However, these mechanisms may put the peripheral tissues (including gastrointestinal tract or muscles) perfusion in danger. Maintaining adequate cardiac output is the first step of pGDT, but may endanger local flow through some organs when not coupled with adequate perfusion pressure. Currently number of large-scale retrospective studies exist to support that even short periods of hypotension are associated with postoperative complications (153-156). In one prospective RCT maintaining adequate perfusion pressure was associated with improved outcomes in patients with arterial hypertension (157). However, based on data by Saugel et al. (158), defining the adequate individual perfusion pressures is not that straightforward, because widely used pre-anesthesia values are far from being accurate surrogate.
Limitations and adoption roadblock
Even though pGDT is currently based on number of positive RCTs and is proposed by several national (or multinational) guidelines, its adoption into real praxis is still challenging (159,160). Recent surveys among anesthesiologists from diverse countries indicates that “pressure monitoring only” is still the prevailing praxis (160-162). Following reasons are major limits in adoption of pGDT:
- Lack of high-level evidence—though we do possess a number of individual RCTs proving a beneficial effect, their heterogeneity precludes to draw any hard conclusions. Besides, the studies demonstrating more benefit are those smaller, with high risk of bias; while large, multi-centric studies often do not prove this (97,99,108).
- Lack of clear-cut approach—the heterogeneity of approaches, devices and treatment targets precludes giving a clear-cut recommendation in whom, using what device, which variable and which target value should be used (163).
- Uncertain cost-benefit—most of monitoring technologies used for pGDT are associated with non-deniable economic burden, the use of pGDT further increases the demands on treating staff leading to increased economic (and personal) costs. Contrary, the benefit of pGDT is postponed and observed in a large-scale view only (available to hospital administrators). Several studies tried to overcome this by putting the results of different RCTs into economical context (164,165), but on individual basis the economic restrains still exists.
Future perspectives
Based on the current evidence pGDT seems to be a rational concept of perioperative care for intermediate-to-high-risk surgical patients. Further development is necessary to overcome most of the uncertainties and roadblocks. Currently three multicentric studies are ongoing [GAS-ART (143), iPEGASUS (144) and OPTIMISE II], which may put some more light on the effectivity of pGDT approach in the context of current perioperative care. However, several concerns could be raised regarding protocols of these trials. A pragmatic fixed dose of inotrope used in OPTIMISE II, SV maximization in GAS-ART (143) do repeat previous attempts but on larger scale. A true individualization should reflect patient’s long-term normal values and reserves, but such protocol has not been tested yet.
Another factor may further help us to design rational individualized pGDT trials in the future. First, the development of non-invasive monitoring tools may enable us to assess the individual target values much more easily. A cumbersome ambulatory oscillometric cuff blood pressure measurement would be replaceable by some of novel technologies for monitoring not only blood pressure but blood flow as well. Hemodynamic profile of individual patient obtained in preoperative period may set the base for perioperative hemodynamic targets. Second improvement, which is on the way, are novel, more specific parameters for individual determinants of cardiac function. Parameters as dynamic elastance, change in arterial pressure in time during upstroke and other parameters may further improve our understanding of the underlying pathology. Finally, the introduction of artificial intelligence and automated closed-loop systems may further help the implementation of pGDT. Recently marketed algorithm seems to be able accurately predict development of spontaneous hypotension in following 5–10 minutes (166,167). This enables the treating team either to get ready or to pre-react, hypothetically enabling to decrease hypotensive periods. Other much simpler algorithms were tested to enable closed-loop systems for decision support for individualized pGDT (68,168).
Putting these entire improvements together one may propose the further design of pGDT approach:
- Based on large scale studies individual patients or small adequately defined populations may be picked out to have profit out of pGDT intervention. A stepwise approach defined by patient risks, surgical intervention and other variables is advisable.
- Preoperative noninvasive testing of the individual CV (and other) system capacity may help to set the proper individual target values. Active approach may be set up for the high-risk patients.
- Based on our better understanding of CV system artificial intelligence may be incorporated in decision making process helping the treating physician to pick up the treatment of choice by and making further decision more precise and individualized.
Conclusions
pGDT is one of the possibilities to improve postoperative outcome of intermediate-to-high risk surgical patients. Based on current evidence it seems to be associated with decreased postoperative length of stay, number of complications and possibly even mortality (in the high-risk population). However, the current evidence is extremely heterogeneous because of large time-span between individual RCTs, monitoring technologies and treatment targets used.
Acknowledgments
Funding: This work was supported by the program for the Development of Scientific Fields of Charles University (Progres Q39).
Footnote
Conflicts of Interest: J Beneš received honoraria for its long-term scientific collaboration with Edwards Lifesciences Inc., CNSystems Medizintechnik GMBh and Gettinge—Pulsion. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Roth GA, Johnson C, Abajobir A, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1-25. [Crossref] [PubMed]
- Nepogodiev D, Martin J, Biccard B, et al. National Institute for Health Research Global Health Research Unit on Global Surgery D, et al. Global burden of postoperative death. Lancet 2019;393:401. [Crossref] [PubMed]
- Pearse RM, Harrison DA, James P, et al. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care 2006;10:R81. [Crossref] [PubMed]
- Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 2005;242:326-41; discussion 341-3. [PubMed]
- Niskanen MM, Takala JA. Use of resources and postoperative outcome. Eur J Surg 2001;167:643-9. [Crossref] [PubMed]
- Sankar A, Beattie WS, Wijeysundera DN. How can we identify the high-risk patient? Curr Opin Crit Care 2015;21:328-35. [Crossref] [PubMed]
- Chong MA, Wang Y, Berbenetz NM, et al. Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes?: A systematic review and meta-analysis. Eur J Anaesthesiol 2018;35:469-83. [PubMed]
- Kaufmann T, Clement RP, Scheeren TWL, et al. Perioperative goal-directed therapy: A systematic review without meta-analysis. Acta Anaesthesiologica Scandinavica 2018;62:1340-55. [Crossref] [PubMed]
- Benes J, Giglio M, Brienza N, et al. The effects of goal-directed fluid therapy based on dynamic parameters on post-surgical outcome: a meta-analysis of randomized controlled trials. Crit Care 2014;18:584. [Crossref] [PubMed]
- Aya HD, Ster IC, Fletcher N, et al. Pharmacodynamic Analysis of a Fluid Challenge. Crit Care Med 2016;44:880-91. [Crossref] [PubMed]
- Cecconi M, Corredor C, Arulkumaran N, et al. Clinical review: Goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care 2013;17:209. [Crossref] [PubMed]
- Shoemaker WC, Appel PL, Kram HB, et al. Prospective Trial of Supranormal Values of Survivors as Therapeutic Goals in High-Risk Surgical Patients. Chest 1988;94:1176-86. [Crossref] [PubMed]
- Shoemaker WC, Appel PL, Kram HB. Tissue oxygen debt as a determinant of lethal and nonlethal postoperative organ failure. Crit Care Med 1988;16:1117-20. [Crossref] [PubMed]
- Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA 1993;270:2699-707. [Crossref] [PubMed]
- Berlauk JF, Abrams JH, Gilmour IJ, et al. Preoperative optimization of cardiovascular hemodynamics improves outcome in peripheral vascular surgery. A prospective, randomized clinical trial. Ann Surg 1991;214:289-97; discussion 298-9. [Crossref] [PubMed]
- Gattinoni L, Brazzi L, Pelosi P, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med 1995;333:1025-32. [Crossref] [PubMed]
- Alía I, Esteban A, Gordo F, et al. A randomized and controlled trial of the effect of treatment aimed at maximizing oxygen delivery in patients with severe sepsis or septic shock. Chest 1999;115:453-61. [Crossref] [PubMed]
- Hayes MA, Timmins AC, Yau E, et al. Elevation of Systemic Oxygen Delivery in the Treatment of Critically Ill Patients. N Engl J Med 1994;330:1717-22. [Crossref] [PubMed]
- Kern JW, Shoemaker WC. Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med 2002;30:1686-92. [Crossref] [PubMed]
- Meregalli A, Oliveira RP, Friedman G. Occult hypoperfusion is associated with increased mortality in hemodynamically stable, high-risk, surgical patients. Crit Care 2004;8:R60-5. [Crossref] [PubMed]
- Mueller MM, Van Remoortel H, Meybohm P, et al. Patient Blood Management: Recommendations From the 2018 Frankfurt Consensus Conference. JAMA 2019;321:983-97. [Crossref] [PubMed]
- Pinsky MR, Payen D. Functional hemodynamic monitoring. Crit Care 2005;9:566-72. [Crossref] [PubMed]
- Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care 2015;19 Suppl 3:S8. [PubMed]
- Hamilton MA, Cecconi M, Rhodes A. A Systematic Review and Meta-Analysis on the Use of Preemptive Hemodynamic Intervention to Improve Postoperative Outcomes in Moderate and High-Risk Surgical Patients. Anesth Analg 2011;112:1392-402. [Crossref] [PubMed]
- Ackland GL, Iqbal S, Paredes LG, et al. Individualised oxygen delivery targeted haemodynamic therapy in high-risk surgical patients: A multicentre, randomised, double-blind, controlled, mechanistic trial. Lancet Respir Med 2015;3:33-41. [Crossref] [PubMed]
- Bahlmann H, Halldestam I, Nilsson L. Goal-directed therapy during transthoracic oesophageal resection does not improve outcome. Eur J Anaesthesiol 2019;36:153-61. [Crossref] [PubMed]
- Bartha E, Arfwedson C, Imnell A, et al. Randomized controlled trial of goal-directed haemodynamic treatment in patients with proximal femoral fracture. Br J Anaesth 2013;110:545-53. [Crossref] [PubMed]
- Bender JS, Smith-Meek MA, Jones CE. Routine pulmonary artery catheterization does not reduce morbidity and mortality of elective vascular surgery: results of a prospective, randomized trial. Ann Surg 1997;226:229-36; discussion 236-7. [Crossref] [PubMed]
- Benes J, Chytra I, Altmann P, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care 2010;14:R118. [Crossref] [PubMed]
- Benes J, Haidingerova L, Pouska J, et al. Fluid management guided by a continuous non-invasive arterial pressure device is associated with decreased postoperative morbidity after total knee and hip replacement. BMC Anesthesiol 2015;15:148. [Crossref] [PubMed]
- Bisgaard J, Gilsaa T, Rønholm E, et al. Optimising stroke volume and oxygen delivery in abdominal aortic surgery: a randomised controlled trial. Acta Anaesthesiol Scand 2013;57:178-88. [Crossref] [PubMed]
- Bisgaard J, Gilsaa T, Rønholm E, et al. Haemodynamic optimisation in lower limb arterial surgery: room for improvement? Acta Anaesthesiol Scand 2013;57:189-98. [Crossref] [PubMed]
- Bishop MH, Shoemaker WC, Appel PL, et al. Prospective, randomized trial of survivor values of cardiac index, oxygen delivery, and oxygen consumption as resuscitation endpoints in severe trauma. J Trauma 1995;38:780-7. [Crossref] [PubMed]
- Bonazzi M, Gentile F, Biasi GM, et al. Impact of perioperative haemodynamic monitoring on cardiac morbidity after major vascular surgery in low risk patients. A randomised pilot trial. Eur J Vasc Endovasc Surg 2002;23:445-51. [Crossref] [PubMed]
- Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA 1993;270:2699-707. [Crossref] [PubMed]
- Brandstrup B, Svendsen PE, Rasmussen M, et al. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near-maximal stroke volume or zero fluid balance?. Br J Anaesth 2012;109:191-9. [Crossref] [PubMed]
- Broch O, Carstens A, Gruenewald M, et al. Non-invasive hemodynamic optimization in major abdominal surgery: A feasibility study. Minerva Anestesiol 2016;82:1158-69. [PubMed]
- Buettner M, Schummer W, Huettemann E, et al. Influence of systolic-pressure-variation-guided intraoperative fluid management on organ function and oxygen transport. Br J Anaesth 2008;101:194-9. [Crossref] [PubMed]
- Bundgaard-Nielsen M, Jans Ø, Müller RG, et al. Does goal-directed fluid therapy affect postoperative orthostatic intolerance?: A randomized trial. Anesthesiology. 2013;119:813-23. [Crossref] [PubMed]
- Calvo-Vecino JM, Ripollés-Melchor J, Mythen MG, et al. Effect of goal-directed haemodynamic therapy on postoperative complications in low–moderate risk surgical patients: a multicentre randomised controlled trial (FEDORA trial). Br J Anaesth 2018;120:734-44. [Crossref] [PubMed]
- Cecconi M, Fasano N, Langiano N, et al. Goal-directed haemodynamic therapy during elective total hip arthroplasty under regional anaesthesia. Crit Care 2011;15:R132. [Crossref] [PubMed]
- Colantonio L, Claroni C, Fabrizi L, et al. A randomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Gastrointest Surg 2015;19:722-9. [Crossref] [PubMed]
- Conway DH, Mayall R, Abdul-Latif MS, et al. Randomised controlled trial investigating the influence of intravenous fluid titration using oesophageal Doppler monitoring during bowel surgery*. Anaesthesia 2002;57:845-9. [Crossref] [PubMed]
- Correa-Gallego C, Tan KS, Arslan-Carlon V, et al. Goal-Directed Fluid Therapy Using Stroke Volume Variation for Resuscitation after Low Central Venous Pressure-Assisted Liver Resection: A Randomized Clinical Trial. J Am Coll Surg 2015;221:591-601. [Crossref] [PubMed]
- Demirel İ, Bolat E, Altun AY, et al. Efficacy of Goal-Directed Fluid Therapy via Pleth Variability Index During Laparoscopic Roux-en-Y Gastric Bypass Surgery in Morbidly Obese Patients. Obes Surg 2018;28:358-63. [Crossref] [PubMed]
- Donati A, Loggi S, Preiser JC, et al. Goal-directed intraoperative therapy reduces morbidity and length of hospital stay in high-risk surgical patients. Chest 2007;132:1817-24. [Crossref] [PubMed]
- El Sharkawy OA, Refaat EK, Ibraheem AE, et al. Transoesophageal Doppler compared to central venous pressure for perioperative hemodynamic monitoring and fluid guidance in liver resection. Saudi J Anaesth 2013;7:378-86. [Crossref] [PubMed]
- Elgendy MA, Esmat IM, Kassim DY. Outcome of intraoperative goal-directed therapy using Vigileo/FloTrac in high-risk patients scheduled for major abdominal surgeries: A prospective randomized trial. Egypt J Anaesth 2017;33:263-9.. [Crossref]
- Fellahi JL, Brossier D, Dechanet F, et al. Early goal-directed therapy based on endotracheal bioimpedance cardiography: a prospective, randomized controlled study in coronary surgery. J Clin Monit Comput 2015;29:351-8. [Crossref] [PubMed]
- Figus A, Wade RG, Oakey S, et al. Intraoperative esophageal Doppler hemodynamic monitoring in free perforator flap surgery. Ann Plast Surg 2013;70:301-7. [PubMed]
- Fleming A, Bishop M, Shoemaker W, et al. Prospective trial of supranormal values as goals of resuscitation in severe trauma. Arch Surg 1992;127:1175-9; discussion 1179-81. [Crossref] [PubMed]
- Forget P, Lois F, De Kock M. Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth Analg 2010;111:910-4. [PubMed]
- Funk DJ. A randomized controlled trial on the effects of goal-directed therapy on the inflammatory response open abdominal aortic aneurysm repair. Crit Care 2015;19:247. [Crossref] [PubMed]
- Funk D, Bohn J, Mutch W, et al. Goal-directed fluid therapy for microvascular free flap reconstruction following mastectomy: A pilot study. Plast Surg (Oakv) 2015;23:231-4. [Crossref] [PubMed]
- Gan TJ, Soppitt A, Maroof M, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 2002;97:820-6. [Crossref] [PubMed]
- Goepfert MSG, Reuter DA, Akyol D, et al. Goal-directed fluid management reduces vasopressor and catecholamine use in cardiac surgery patients. Intensive Care Med 2007;33:96-103. [Crossref] [PubMed]
- Goepfert MS, Richter HP, Zu Eulenburg C, et al. Individually optimized hemodynamic therapy reduces complications and length of stay in the intensive care unit: a prospective, randomized controlled trial. Anesthesiology 2013;119:824-36. [Crossref] [PubMed]
- Gómez-Izquierdo JC, Trainito A, Mirzakandov D, et al. Goal-directed Fluid Therapy Does Not Reduce Primary Postoperative Ileus after Elective Laparoscopic Colorectal Surgery: A Randomized Controlled Trial. Anesthesiology 2017;127:36-49. [Crossref] [PubMed]
- Hand WR, Stoll WD, McEvoy MD, et al. Intraoperative goal-directed hemodynamic management in free tissue transfer for head and neck cancer. Head Neck 2016;38 Suppl 1:E1974-80. [Crossref] [PubMed]
- Harten J, Crozier JE, McCreath B, et al. Effect of intraoperative fluid optimisation on renal function in patients undergoing emergency abdominal surgery: a randomised controlled pilot study (ISRCTN 11799696). Int J Surg 2008;6:197-204. [Crossref] [PubMed]
- Hasanin A, Mourad KH, Farouk I, et al. The Impact of Goal-Directed Fluid Therapy in Prolonged Major Abdominal Surgery on Extravascular Lung Water and Oxygenation: A Randomized Controlled Trial. Open Access Maced J Med Sci 2019;7:1276-81. [Crossref] [PubMed]
- Challand C, Struthers R, Sneyd JR, et al. Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Br J Anaesth 2012;108:53-62. [Crossref] [PubMed]
- Chytra I, Pradl R, Bosman R, et al. Esophageal Doppler-guided fluid management decreases blood lactate levels in multiple-trauma patients: a randomized controlled trial. Crit Care 2007;11:R24. [Crossref] [PubMed]
- Jain AK, Khan AM. Stroke volume variation as a guide for fluid resuscitation in patients undergoing large-volume liposuction. Plast Reconstr Surg 2012;130:462e-9e. [Crossref] [PubMed]
- Jammer I, Ulvik A, Erichsen C, et al. Does central venous oxygen saturation-directed fluid therapy affect postoperative morbidity after colorectal surgery? A randomized assessor-blinded controlled trial. Anesthesiology 2010;113:1072-80. [Crossref] [PubMed]
- Jhanji S, Vivian-Smith A, Lucena-Amaro S, et al. Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: a randomised controlled trial. Crit Care 2010;14:R151. [Crossref] [PubMed]
- Jones C, Kelliher L, Dickinson M, et al. Randomized clinical trial on enhanced recovery versus standard care following open liver resection. Br J Surg 2013;100:1015-24. [Crossref] [PubMed]
- Joosten A, Huynh T, Suehiro K, et al. Goal-Directed fluid therapy with closed-loop assistance during moderate risk surgery using noninvasive cardiac output monitoring: A pilot study. Br J Anaesth 2015;114:886-92. [Crossref] [PubMed]
- Kapoor PM, Kakani M, Chowdhury U, et al. Early goal-directed therapy in moderate to high-risk cardiac surgery patients. Ann Card Anaesth 2008;11:27-34. [Crossref] [PubMed]
- Kapoor PM, Magoon R, Rawat R, et al. Perioperative utility of goal-directed therapy in high-risk cardiac patients undergoing coronary artery bypass grafting: "A clinical outcome and biomarker-based study Ann Card Anaesth 2016;19:638-82. [Crossref] [PubMed]
- Kapoor PM, Magoon R, Rawat RS, et al. Goal-directed therapy improves the outcome of high-risk cardiac patients undergoing off-pump coronary artery bypass. Ann Card Anaesth 2017;20:83-9. [Crossref] [PubMed]
- Kaufmann KB, Stein L, Bogatyreva L, et al. Oesophageal Doppler guided goal-directed haemodynamic therapy in thoracic surgery - a single centre randomized parallel-arm trial. Br J Anaesth 2017;118:852-61. [Crossref] [PubMed]
- Kim HJ, Kim EJ, Lee HJ, et al. Effect of goal-directed haemodynamic therapy in free flap reconstruction for head and neck cancer. Acta Anaesthesiol Scand 2018;62:903-14. [Crossref] [PubMed]
- Kumar L, Kanneganti YS, Rajan S. Outcomes of implementation of enhanced goal directed therapy in high-risk patients undergoing abdominal surgery. Indian J Anaesth 2015;59:228-33. [Crossref] [PubMed]
- Kumar L, Rajan S, Baalachandran R. Outcomes associated with stroke volume variation versus central venous pressure guided fluid replacements during major abdominal surgery. J Anaesthesiol Clin Pharmacol 2016;32:182-6. [Crossref] [PubMed]
- Lai CW, Starkie T, Creanor S, et al. Randomized controlled trial of stroke volume optimization during elective major abdominal surgery in patients stratified by aerobic fitness. Br J Anaesth 2015;115:578-89. [Crossref] [PubMed]
- Lee S, Lee SH, Chang BC, et al. Efficacy of Goal-Directed Therapy Using Bioreactance Cardiac Output Monitoring after Valvular Heart Surgery. Yonsei Med J 2015;56:913-20. [Crossref] [PubMed]
- Lenkin AI, Kirov MY, Kuzkov VV, et al. Comparison of goal-directed hemodynamic optimization using pulmonary artery catheter and transpulmonary thermodilution in combined valve repair: a randomized clinical trial. Crit Care Res Pract 2012;2012:821218. [Crossref] [PubMed]
- Li B, Zhang L, Zhang S, et al. Application of ultrasound in goal-directed fluid management of anesthesia in elderly patients. Biomedical Research 2017.S482-6.
- Liang M, Li Y, Lin L, et al. Effect of goal-directed fluid therapy on the prognosis of elderly patients with hypertension receiving plasmakinetic energy transurethral resection of prostate. Int J Clin Exp Med 2017;10:1290-6.
- Liu TJ, Zhang JC, Gao XZ, et al. Clinical research of goal-directed fluid therapy in elderly patients with radical resection of bladder cancer. J Cancer Res Ther 2018;14:S173-S179. [Crossref] [PubMed]
- Lobo SM, Salgado PF, Castillo VG, et al. Effects of maximizing oxygen delivery on morbidity and mortality in high-risk surgical patients. Crit Care Med 2000;28:3396-404. [Crossref] [PubMed]
- Lobo SM, Lobo FR, Polachini CA, et al. Prospective, randomized trial comparing fluids and dobutamine optimization of oxygen delivery in high-risk surgical patients (ISRCTN42445141). Crit Care 2006;10:R72. [Crossref] [PubMed]
- Lopes MR, Oliveira MA, Pereira VO, et al. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit Care 2007;11:R100. [Crossref] [PubMed]
- Luo J, Xue J, Liu J, et al. Goal-directed fluid restriction during brain surgery: a prospective randomized controlled trial. Ann Intensive Care 2017;7:16. [Crossref] [PubMed]
- Mayer J, Boldt J, Mengistu AM, et al. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trial. Crit Care 2010;14:R18. [Crossref] [PubMed]
- McKendry M, McGloin H, Saberi D, et al. Randomised controlled trial assessing the impact of a nurse delivered, flow monitored protocol for optimisation of circulatory status after cardiac surgery. BMJ 2004;329:258. [Crossref] [PubMed]
- McKenny M, Conroy P, Wong A, et al. A randomised prospective trial of intra-operative oesophageal Doppler-guided fluid administration in major gynaecological surgery. Anaesthesia 2013;68:1224-31. [Crossref] [PubMed]
- Mikor A, Trásy D, Németh MF, et al. Continuous central venous oxygen saturation assisted intraoperative hemodynamic management during major abdominal surgery: a randomized, controlled trial. BMC Anesthesiol 2015;15:82. [Crossref] [PubMed]
- Moppett IK, Rowlands M, Mannings A, et al. LiDCO-based fluid management in patients undergoing hip fracture surgery under spinal anaesthesia: a randomized trial and systematic review. Br J Anaesth 2015;114:444-59. [Crossref] [PubMed]
- Mythen MG, Webb AR. Perioperative Plasma Volume Expansion Reduces the Incidence of Gut Mucosal Hypoperfusion During Cardiac Surgery. Arch Surg 1995;130:423-9. [Crossref] [PubMed]
- Noblett SE, Snowden CP, Shenton BK, et al. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg 2006;93:1069-76. [Crossref] [PubMed]
- Osawa EA, Rhodes A, Landoni G, et al. Effect of Perioperative Goal-Directed Hemodynamic Resuscitation Therapy on Outcomes Following Cardiac Surgery: A Randomized Clinical Trial and Systematic Review. Crit Care Med 2016;44:724-33. [PubMed]
- Parke RL, McGuinness SP, Gilder E, et al. A randomised feasibility study to assess a novel strategy to rationalise fluid in patients after cardiac surgery. Br J Anaesth 2015;115:45-52. [Crossref] [PubMed]
- Pavlovic G, Diaper J, Ellenberger C, et al. Impact of early haemodynamic goal-directed therapy in patients undergoing emergency surgery: an open prospective, randomised trial. J Clin Monit Comput 2016;30:87-99. [Crossref] [PubMed]
- Pearse R, Dawson D, Fawcett J, et al. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial (ISRCTN38797445). Crit Care 2005;9:R687-93. [Crossref] [PubMed]
- Pearse RM, Harrison DA, MacDonald N, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 2014;311:2181-90. [Crossref] [PubMed]
- Peng K, Li J, Cheng H, et al. Goal-directed fluid therapy based on stroke volume variations improves fluid management and gastrointestinal perfusion in patients undergoing major orthopedic surgery. Med Princ Pract 2014;23:413-20. [Crossref] [PubMed]
- Pestaña D, Espinosa E, Eden A, et al. Perioperative goal-directed hemodynamic optimization using noninvasive cardiac output monitoring in major abdominal surgery: A prospective, randomized, multicenter, pragmatic trial: POEMAS study (PeriOperative goal-directed thErapy in Major Abdominal Surg. Anesth Analg 2014;119:579-87. [Crossref] [PubMed]
- Phan TD, D’Souza B, Rattray MJ, et al. A randomised controlled trial of fluid restriction compared to oesophageal Doppler-guided goal-directed fluid therapy in elective major colorectal surgery within an Enhanced Recovery After Surgery program. Anaesth Intensive Care 2014;42:752-60. [Crossref] [PubMed]
- Picard J, Bedague D, Bouzat P, et al. Oesophageal Doppler to optimize intraoperative haemodynamics during prone position. A randomized controlled trial. Anaesth Crit Care Pain Med 2016;35:255-60. [Crossref] [PubMed]
- Pillai P, McEleavy I, Gaughan M, et al. A double-blind randomized controlled clinical trial to assess the effect of doppler optimized intraoperative fluid management on outcome following radical cystectomy. J Urol 2011;186:2201-6. [Crossref] [PubMed]
- Pölönen P, Ruokonen E, Hippeläinen M, et al. Prospective, Randomized Study of Goal-Oriented Hemodynamic Therapy in Cardiac Surgical Patients. Anesth Analg 2000;90:1052-9. [Crossref] [PubMed]
- Pösö T, Winsö O, Aroch R, et al. Perioperative fluid guidance with transthoracic echocardiography and pulse-contour device in morbidly obese patients. Obes Surg 2014;24:2117-25. [Crossref] [PubMed]
- Ramsingh DS, Sanghvi C, Gamboa J, et al. Outcome impact of goal directed fluid therapy during high risk abdominal surgery in low to moderate risk patients: a randomized controlled trial. J Clin Monit Comput 2013;27:249-57. [Crossref] [PubMed]
- Reisinger KW, Willigers HM, Jansen J, et al. Doppler-guided goal-directed fluid therapy does not affect intestinal cell damage but increases global gastrointestinal perfusion in colorectal surgery: a randomized controlled trial. Colorectal Dis 2017;19:1081-91. [Crossref] [PubMed]
- Salzwedel C, Puig J, Carstens A, et al. Perioperative goal-directed hemodynamic therapy based on radial arterial pulse pressure variation and continuous cardiac index trending reduces postoperative complications after major abdominal surgery: a multi-center, prospective, randomized study. Crit Care 2013;17:R191. [Crossref] [PubMed]
- Sandham JD, Hull RD, Brant RF, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 2003;348:5-14. [Crossref] [PubMed]
- Senagore AJ, Emery T, Luchtefeld M, et al. Fluid management for laparoscopic colectomy: a prospective, randomized assessment of goal-directed administration of balanced salt solution or hetastarch coupled with an enhanced recovery program. Dis Colon Rectum 2009;52:1935-40. [Crossref] [PubMed]
- Sethi A, Debbarma M, Narang N, et al. Impact of Targeted Preoperative Optimization on Clinical Outcome in Emergency Abdominal Surgeries: A Prospective Randomized Trial. Anesth Essays Res 2018;12:149-54. [Crossref] [PubMed]
- Scheeren TW, Wiesenack C, Gerlach H, et al. Goal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients: a prospective randomized multicentre study. J Clin Monit Comput 2013;27:225-33. [Crossref] [PubMed]
- Schmid S, Kapfer B, Heim M, et al. Algorithm-guided goal-directed haemodynamic therapy does not improve renal function after major abdominal surgery compared to good standard clinical care: a prospective randomised trial. Crit Care 2016;20:50. [Crossref] [PubMed]
- Schultz RJ, Whitfield GF, LaMura JJ, et al. The role of physiologic monitoring in patients with fractures of the hip. J Trauma 1985;25:309-16. [Crossref] [PubMed]
- Sinclair S, James S, Singer M. Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. BMJ 1997;315:909-12. [Crossref] [PubMed]
- Smetkin AA, Kirov MY, Kuzkov VV, et al. Single transpulmonary thermodilution and continuous monitoring of central venous oxygen saturation during off-pump coronary surgery. Acta Anaesthesiol Scand 2009;53:505-14. [Crossref] [PubMed]
- Stens J, Hering JP, van der Hoeven CWP, et al. The added value of cardiac index and pulse pressure variation monitoring to mean arterial pressure-guided volume therapy in moderate-risk abdominal surgery (COGUIDE): a pragmatic multicentre randomised controlled trial. Anaesthesia 2017;72:1078-87. [Crossref] [PubMed]
- Szakmany T, Toth I, Kovacs Z, et al. Effects of volumetric vs. pressure-guided fluid therapy on postoperative inflammatory response: a prospective, randomized clinical trial. Intensive Care Med 2005;31:656-63. [Crossref] [PubMed]
- Szturz P, Folwarczny P, Kula R, et al. Multi-parametric functional hemodynamic optimization improves postsurgical outcome after intermediate risk open gastrointestinal surgery: a randomized controlled trial. Minerva Anestesiol 2019;85:244-54. [Crossref] [PubMed]
- Thomson R, Meeran H, Valencia O, et al. Goal-directed therapy after cardiac surgery and the incidence of acute kidney injury. J Crit Care 2014;29:997-1000. [Crossref] [PubMed]
- Torregiani G, Claroni C, Covotta M, et al. Impact of a goal-directed fluid therapy on length of hospital stay and costs of hepatobiliarypancreatic surgery: a prospective observational study. J Comp Eff Res 2018;7:1171-9. [Crossref] [PubMed]
- Ueno S, Tanabe G, Yamada H, et al. Response of patients with cirrhosis who have undergone partial hepatectomy to treatment aimed at achieving supranormal oxygen delivery and consumption. Surgery 1998;123:278-86. [Crossref] [PubMed]
- Valentine RJ, Duke ML, Inman MH, et al. Effectiveness of pulmonary artery catheters in aortic surgery: a randomized trial. J Vasc Surg 1998;27:203-11; discussion 211-2. [Crossref] [PubMed]
- Van der Linden PJ, Dierick A, Wilmin S, et al. A randomized controlled trial comparing an intraoperative goal-directed strategy with routine clinical practice in patients undergoing peripheral arterial surgery. Eur J Anaesthesiol 2010;27:788-93. [Crossref] [PubMed]
- Velmahos GC, Demetriades D, Shoemaker WC, et al. Endpoints of resuscitation of critically injured patients: normal or supranormal? A prospective randomized trial. Ann Surg 2000;232:409-18. [Crossref] [PubMed]
- Venn R, Steele A, Richardson P, et al. Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Br J Anaesth 2002;88:65-71. [Crossref] [PubMed]
- Wakeling HG, McFall MR, Jenkins CS, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth 2005;95:634-42. [Crossref] [PubMed]
- Wenkui Y, Ning L, Jianfeng G, et al. Restricted peri-operative fluid administration adjusted by serum lactate level improved outcome after major elective surgery for gastrointestinal malignancy. Surgery 2010;147:542-52. [Crossref] [PubMed]
- Wilson J, Woods I, Fawcett J, et al. Reducing the risk of major elective surgery: randomised controlled trial of preoperative optimisation of oxygen delivery. BMJ 1999;318:1099-103. [Crossref] [PubMed]
- Wu J, Ma Y, Wang T, et al. Goal-directed fluid management based on the auto-calibrated arterial pressure-derived stroke volume variation in patients undergoing supratentorial neoplasms surgery. Int J Clin Exp Med 2017;10:3106-14.
- Xiao W, Duan Q, Zhao L, et al. Goal-directed fluid therapy may improve hemodynamic stability in parturient women under combined spinal epidural anesthesia for cesarean section and newborn well-being. J Obstet Gynaecol Res 2015;41:1547-55. [Crossref] [PubMed]
- Xu H, Shu SH, Wang D, et al. Goal-directed fluid restriction using stroke volume variation and cardiac index during one-lung ventilation: A randomized controlled trial. J Thorac Dis 2017;9:2992-3004. [Crossref] [PubMed]
- Yassen AM. Pressure versus volume indices to guide fluid infusion early after living donor liver transplantation: A prospective randomized controlled trial. Egypt J Anaesth 2012;28:223-30. [Crossref]
- Yin K, Ding J, Wu Y, et al. Goal-directed fluid therapy based on noninvasive cardiac output monitor reduces postoperative complications in elderly patients after gastrointestinal surgery: A randomized controlled trial. Pak J Med Sci 2018;34:1320-5. [Crossref] [PubMed]
- Yu Y, Dong J, Xu Z, et al. Pleth variability index-directed fluid management in abdominal surgery under combined general and epidural anesthesia. J Clin Monit Comput 2015;29:47-52. [Crossref] [PubMed]
- Zakhaleva J, Tam J, Denoya PI, et al. The impact of intravenous fluid administration on complication rates in bowel surgery within an enhanced recovery protocol: a randomized controlled trial. Colorectal Dis 2013;15:892-9. [Crossref] [PubMed]
- Zhang J, Chen C, Lei X, et al. Goal-directed fluid optimization based on stroke volume variation and cardiac index during one-lung ventilation in patients undergoing thoracoscopy lobectomy operations: a pilot study. Clinics 2013;68:1065-70. [Crossref] [PubMed]
- Zhang J, Qiao H, He Z, et al. Intraoperative fluid management in open gastrointestinal surgery: goal-directed versus restrictive. Clinics 2012;67:1149-55. [Crossref] [PubMed]
- Zheng H, Guo H, Ye JR, et al. Goal-directed fluid therapy in gastrointestinal surgery in older coronary heart disease patients: randomized trial. World J Surg 2013;37:2820-9. [Crossref] [PubMed]
- Ziegler DW, Wright JG, Choban PS, et al. A prospective randomized trial of preoperative “optimization” of cardiac function in patients undergoing elective peripheral vascular surgery. Surgery 1997;122:584-92. [Crossref] [PubMed]
- Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 2002;121:2000-8. [Crossref] [PubMed]
- National Institute for Health and Clinical Excellence. Medical technologies guidance MTG3: CardioQ-ODM oesophageal doppler monitor. 2011. Available online: http://www.nice.org.uk/MTG3. Accessed 4 Jan 2017.
- Schober P, Loer SA, Schwarte LA. Perioperative Hemodynamic Monitoring with Transesophageal Doppler Technology. Anesth Analg 2009;109:340-53. [Crossref] [PubMed]
- Voldby AW, Aaen AA, Møller AM, et al. Goal-directed fluid therapy in urgent GAstrointestinal Surgery—study protocol for A Randomised multicentre Trial: The GAS-ART trial. BMJ Open 2018;8:e022651. [Crossref] [PubMed]
- Funcke S, Saugel B, Koch C, et al. Individualized, perioperative, hemodynamic goal-directed therapy in major abdominal surgery (iPEGASUS trial): study protocol for a randomized controlled trial. Trials 2018;19:273. [Crossref] [PubMed]
- Thiele RH, Bartels K, Gan TJ. Cardiac Output Monitoring. Crit Care Med 2015;43:177-85. [Crossref] [PubMed]
- Joosten A, Desebbe O, Suehiro K, et al. Accuracy and precision of non-invasive cardiac output monitoring devices in perioperative medicine: a systematic review and meta-analysis†. BJA Br J Anaesth 2017;118:298-310. [Crossref] [PubMed]
- Saugel B, Cecconi M, Wagner JY, et al. Noninvasive continuous cardiac output monitoring in perioperative and intensive care medicine. Br J Anaesth 2015;114:562-75. [Crossref] [PubMed]
- Benes J, Kasal E. New Fully Non-invasive Hemodynamic Monitoring Technologies: Groovy or Paltry Tools. In: Vincent JL. editor. Annual Update in Intensive Care and Emergency Medicine 2015. Cham: Springer International Publishing, 2015:249-58.
- Kuper M, Gold SJ, Callow C, et al. Intraoperative fluid management guided by oesophageal Doppler monitoring. BMJ 2011;342:d3016. [Crossref] [PubMed]
- Marik PE, Cavallazzi R, Vasu T, et al. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: A systematic review of the literature*. Crit Care Med 2009;37:2642-7. [Crossref] [PubMed]
- Zhang Z, Lu B, Sheng X, et al. Accuracy of stroke volume variation in predicting fluid responsiveness: a systematic review and meta-analysis. J Anesth 2011;25:904-16. [Crossref] [PubMed]
- Yang X, Du B. Does pulse pressure variation predict fluid responsiveness in critically ill patients? A systematic review and meta-analysis. Crit Care 2014;18:650. [Crossref] [PubMed]
- Wesselink EM, Kappen TH, Torn HM, et al. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth 2018;121:706-21. [Crossref] [PubMed]
- Walsh M, Kurz A, Turan A, et al. Relationship between Intraoperative Mean Arterial Pressure and Clinical Outcomes after Noncardiac Surgery. Anesthesiology 2013;119:507-15. [Crossref] [PubMed]
- Salmasi V, Maheshwari K, Yang D, et al. Relationship between Intraoperative Hypotension, Defined by Either Reduction from Baseline or Absolute Thresholds, and Acute Kidney and Myocardial Injury after Noncardiac Surgery. Anesthesiology 2017;126:47-65. [Crossref] [PubMed]
- Sun LY, Wijeysundera DN, Tait GA, et al. Association of Intraoperative Hypotension with Acute Kidney Injury after Elective Noncardiac Surgery. Anesthesiology 2015;123:515-23. [Crossref] [PubMed]
- Futier E, Lefrant JY, Guinot PG, et al. Effect of Individualized vs Standard Blood Pressure Management Strategies on Postoperative Organ Dysfunction Among High-Risk Patients Undergoing Major Surgery. JAMA 2017;318:1346. [Crossref] [PubMed]
- Saugel B, Reese PC, Sessler DI, et al. Automated Ambulatory Blood Pressure Measurements and Intraoperative Hypotension in Patients Having Noncardiac Surgery with General Anesthesia. Anesthesiology 2019;131:74-83. [Crossref] [PubMed]
- Srinivasa S, Kahokehr A, Soop M, et al. Goal-directed fluid therapy- a survey of anaesthetists in the UK, USA, Australia and New Zealand. BMC Anesthesiol 2013;13:5. [Crossref] [PubMed]
- Suehiro K, Tanaka K, Mukai A, et al. Hemodynamic monitoring and management in high-risk surgery: a survey among Japanese anesthesiologists. J Anesth 2016;30:526-9. [Crossref] [PubMed]
- Cannesson M, Pestel G, Ricks C, et al. Hemodynamic monitoring and management in patients undergoing high risk surgery: a survey among North American and European anesthesiologists. Crit Care 2011;15:R197. [Crossref] [PubMed]
- Chen G, Zuo Y, Yang L, et al. Hemodynamic monitoring and management of patients undergoing high-risk surgery: a survey among Chinese anesthesiologists. J Biomed Res 2014;28:376-82. [PubMed]
- Saugel B, Kouz K, Scheeren TWL. The '5 Ts' of perioperative goal-directed haemodynamic therapy. Br J Anaesth 2019;123:103-7. [Crossref] [PubMed]
- Benes J, Zatloukal J, Simanova A, et al. Cost analysis of the stroke volume variation guided perioperative hemodynamic optimization – an economic evaluation of the SVVOPT trial results. BMC Anesthesiol 2014;14:40. [Crossref] [PubMed]
- Mowatt G, Houston G, Hernández R, et al. Systematic review of the clinical effectiveness and cost-effectiveness of oesophageal Doppler monitoring in critically ill and high-risk surgical patients. Health Technol Assess 2009;13:iii-iv, ix-xii, 1-95.
- Hatib F, Jian Z, Buddi S, et al. Machine-learning Algorithm to Predict Hypotension Based on High-fidelity Arterial Pressure Waveform Analysis. Anesthesiology 2018;129:663-74. [Crossref] [PubMed]
- Davies SJ, Vistisen ST, Jian Z, et al. Ability of an Arterial Waveform Analysis-Derived Hypotension Prediction Index to Predict Future Hypotensive Events in Surgical Patients. Anesth Analg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Rinehart J, Lee C, Canales C, et al. Closed-Loop Fluid Administration Compared to Anesthesiologist Management for Hemodynamic Optimization and Resuscitation During Surgery. Anesth Analg 2013;117:1119-29. [Crossref] [PubMed]
Cite this article as: Zatloukal J, Pouska J, Beneš J. Perioperative goal directed therapy—current view. J Emerg Crit Care Med 2019;3:49.


