A retrospective study of the effectiveness of ulinastatin in the treatment of sepsis
Introduction
With developments in modern medicine, sepsis remains a worldwide medical challenge because of related high costs, morbidity, and mortality. Although the recognition and management of sepsis have advanced, mortality among patients with sepsis remains over 30%, and may climb to 60% when septic shock occurs (1). The updated definition of sepsis is “life-threatening organ dysfunction caused by a dysregulated host response to infection” (2). This new definition focuses not only on the excessive inflammatory response and immune suppression, but also on organ function. Ulinastatin (UTI) is a urinary trypsin inhibitor first discovered in urine in 1909 by Bauer and Reich. UTI is a natural serine protease inhibitor present in human blood and urine and composed of 143 amino acids. Because it has two Kunitz-type domains (3), UTI can inhibit the function of various enzymes. The human body contains endogenous UTI; levels increase with infection, operation, or shock. UTI has been shown to reduce the inflammatory response, suppress lymphocyte apoptosis, and improve the microcirculation, protecting the organs (4,5). However, most patients in previous studies (more than 800 patients) were treated with UTI in combination with thymosin alpha-1, making interpretation of the independent therapeutic potency of UTI difficult (6). Further studies are needed to confirm the effectiveness of UTI without thymosin alpha-1 in the treatment of sepsis. A prospective, double-blind, randomized, placebo-controlled trial of UTI in patients with severe sepsis showed that intravenous administration of UTI at a dose of 200,000 U twice daily for 5 days was associated with a reduction in 28-day all-cause mortality (the primary endpoint) to 7.3%, compared with 20.3% in the placebo control group (7). In that study, the mean patient age was below 40 years and the mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score was under 15, values that obviously differ from those of patients in Chinese intensive care units (ICUs). Some recent researches indicate that treatment with UTI could reduce the 28-day mortality in critically ill septic patients (8,9). But it does not explore the different dose of UTI affect to the mortality of patients. In this study, we aimed to explore the effectiveness of UTI in the treatment of sepsis and to determine the proper usage by using a Cox proportional hazards model.
Methods
This study included 295 patients with sepsis treated in the ICU of the First Affiliated Hospital of Xi’an Jiaotong University from January 1, 2012 to December 31, 2017. All patients included in this study met the criteria of the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), including confirmed or suspected infection and an acute change in total Sequential Organ Failure Assessment (SOFA) scores of ≥2 points (2). Patients under the age of 18 years and pregnant patients were excluded. All enrolled patients were divided into two groups, the UTI group and control group, based on UTI use during ICU stay. There were 234 patients in the UTI group and 61 in the control group. Using dose of the patients in UTI group were determined by professors according to the clinical situation of patients. Some studies indicate that UTI has dose-dependent effects, and it’s safety to use a higher dose of UTI in adult healthy Chinese volunteers (10,11).
All potential factors relating to outcomes were considered, including age, sex, underlying diseases [diabetes mellitus, hypertension, coronary heart disease (CHD), cerebral hemorrhage, cerebral infarction, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), tumor, and trauma], body temperature, blood pressure, mean arterial pressure, APACHE II score, SOFA score, Glasgow coma scale score, laboratory values (all the lab variables are chosen the worst values among 48 h after admission to the ICU), infection source, culture results (including culture of blood, sputum, bronchoalveolar lavage fluid, urine, pleural effusion, ascites, skin eruption, and catheter tip), and main interventions (including vasopressors, antibiotics, corticosteroids, human albumin, thymosin, blood purification, and mechanical ventilation). We follow the STROBE checklist to report the work (Table S1).
Full table
Statistical analysis
Categorical variables were reported as numbers and proportions and were analyzed with Pearson’s chi-square or Fisher’s exact test, as appropriate. Continuous variables that met the normality test were reported as means and standard deviations and were analyzed with an independent-sample t-test, or were reported as medians and interquartile ranges and analyzed with the Mann-Whitney U test (12). Variables that were statistically significant in univariate analysis were included in a multivariate Cox proportional hazards model (13). All statistical analyses were performed with SPSS 22.0 software. A P value <0.05 was considered statistically significant.
Results
Baseline characteristics in the UTI and control groups
Among the 295 patients, 181 were men and 14 were women. Mean patient age was 59.98±17.78 years; minimum APACHE II score was 20.02±8.13 and minimum SOFA score was 7.48±3.73 in the 24 h after diagnosis. Infection most commonly originated in the lungs (70.85%), followed by abdominal cavity (37.63%), urine (23.05%), skin (10.17%) and other (7.12%). Only 23.73% of blood cultures were positive. The most commonly cultured organisms were Gram-negative bacteria (40.00%), followed by Gram-positive bacteria (26.10%) and fungi (17.63%).
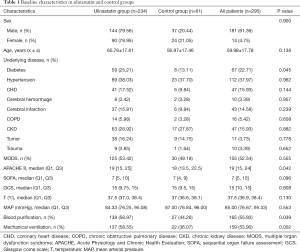
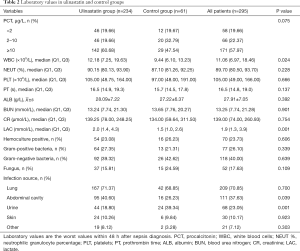
There were 234 patients in the UTI group and 61 in the control group. The UTI group had higher APACHE II scores, WBC counts, and lactate values than the control group, and was more likely to undergo blood purification and mechanical ventilation. There was no significant difference between the groups in sex, age, underlying disease, or most laboratory indices (Tables 1-3).
Full table
Full table

Full table
Cox analysis of UTI effectiveness
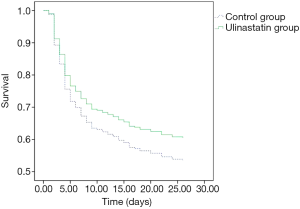
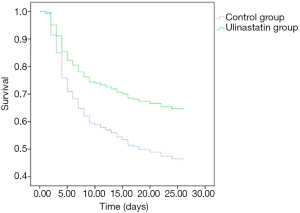
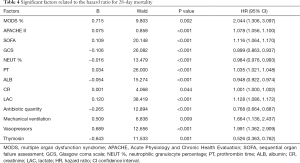
There was no significant difference in 28-day mortality between the UTI group (39.32%) and control group (47.54%, P=0.245). Univariate Cox regression analysis showed that the hazard ratio (HR) for mortality in the UTI group was 0.805 [95% confidence interval (CI): 0.530–1.222, P=0.308]. The survival curve is shown in Figure 1. After adjusting for confounding factors that were statistically significant in univariate analysis (Table 4) with a Cox proportional hazards model, the adjusted HR for mortality with UTI treatment was 0.560 (95% CI: 0.346–0.906, P=0.018). This finding provides evidence that UTI could reduce the 28-day mortality risk by 44% among septic patients. Outcomes are shown in Figure 2.
Full table
Cox regression analysis on the usage of UTI
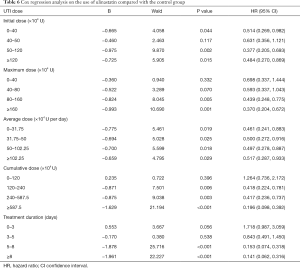
In the UTI group, the average cumulative UTI dose was 4,845,300 U (range, 200,000–43,600,000 U); the average duration of UTI use was 6.29 days (range, 1–25 days) (Table 5). The initial dose, maximum dose, mean dose, cumulative dose, and days of treatment were grouped according to quartiles. The resulting Cox regression analysis showed that the protective effect of UTI against 28-day mortality increased with increasing maximum dose, increasing cumulative dose, and longer duration of treatment. When the dose of UTI is [0–40] ×104 U, the adjusted HR for mortality with UTI treatment was 0.698 (95% CI: 0.337–1.444, P=0.332), when the dose of UTI is more than 160×104 U, the adjusted HR for mortality with UTI treatment was 0.370 (95% CI: 0.204–0.672, P=0.001), UTI had no significant effect on the relative risk (RR) of death when the cumulative dose was less than 1,200,000 U and the duration of use was less than 5 days (Table 6). We choose the cumulative dose of UTI to illustrate the effect of treatment, because we must continuously use UTI to keep the effectiveness of UTI, but not the once reaction.

Full table
Full table
Discussion
Sepsis remains a leading cause of death in the ICU, because the excessive inflammatory response damages vessels, endothelium, and organs. Many studies have established that UTI, a trypsin inhibitor, can inhibit inflammatory responses and oxidative stress, reducing organ damage by regulating some cytokine pathways. Most studies have found that UTI decreased ICU mortality, improved oxygenation, and decreased duration of stay. UTI can also reduce mortality among septic patients in a dose-dependent manner. However, most patients in previous studies (more than 800 patients) were treated with UTI in combination with thymosin alpha-1, making interpretation of the independent therapeutic potency of UTI difficult (5,6,9,14,15). A recent meta-analysis on UTI showed that the 28-day mortality rate was significantly lower in the UTI group than the control group (26.9% vs. 41.6%, RR =0.64, 95% CI: 0.54–0.75, P<0.01) (16). However, the eight studies included in the meta-analysis were not of high quality, and two were of low quality, which reduced the reliability of the results. A prospective, double-blind, multi-center randomized controlled trial in India (7) was included in this meta-analysis. That study reported that UTI significantly reduced the 28-day mortality rate of patients with sepsis (7.3% vs. 20.3%, P=0.045). In that study, the mean age was under 40 years and the mean APACHE II score was under 15; these values are obviously different from those among patients in ICUs in China. Therefore, we collected the clinical data of patients with sepsis in the ICU of the First Hospital Affiliated to Xi’an Jiaotong University from January 1, 2012 to December 31, 2017, aiming to explore the effectiveness of UTI in septic patients and to determine the proper usage by using a Cox proportional hazards model.
Among all 295 patients, 234 (79.32%) received UTI. Patients in the UTI group had higher APACHE II scores, WBC counts, and lactate levels, and were more likely to receive blood purification and mechanical ventilation than patients in the control group. The average initial UTI dose was 824,400 U/day; the average cumulative dose was 4,845,300 U, which was much higher than the normal dosage of 100,000 U to 200,000 U per dose, three times per day. However, the standard deviation and maximum and minimum values show that there were large differences in the use of UTI, which may relate to the differences in disease severity among patients, and the lack of generally recognized treatment guidelines. As known to all, each drug requires a daily dose, but UTI is used to treat sepsis, which usually needs to maintain over a period of time, so we considered it is necessary to understand the duration of use and the cumulative dose and to discover the effect on patient mortality.
There was no significant difference in 28-day mortality between the UTI group and the control group (UTI: 39.32%, control: 47.54%; χ2=1.353, P=0.245). Univariate Cox regression analysis showed that the RR for 28-day mortality with UTI treatment was 0.805 (95% CI: 0.530–1.222, P=0.308). After adjustment with the Cox proportional hazards model for variables that were significant in univariate analysis, the adjusted RR for 28-day mortality with UTI treatment was 0.560 (95% CI: 0.346–0.906, P=0.018). This finding indicates that UTI can effectively reduce the risk of 28-day mortality by 44% in patients with sepsis. Cox regression analysis on the usage of UTI showed that increased maximum dose, higher cumulative dose, and longer duration of use of UTI were associated with an increased protective effect against 28-day mortality.
Given the Cox multivariate regression analysis results, we can draw the following conclusions: (I) in treatment of sepsis, UTI could be beneficial to patients by significantly reducing the risk of death. (II) The effect of UTI in reducing the risk of death may be related to the dose and duration of treatment. In this study, the relative mortality risk in patients treated with UTI decreased with an increase in the maximum dose, cumulative dose, and duration of treatment. When the cumulative dose was less than 1,200,000 IU and the duration was less than 5 days, UTI had no significant effect on the RR of death in patients with sepsis.
The multivariate Cox regression analysis in this study was carried out for patients with sepsis who received UTI. However, the baseline data were different between the UTI and control groups because this was a retrospective study, which may reduce comparability between the groups. Although we considered 52 variables that may affect outcomes, it is possible that some variables were not included, so it is difficult to obtain clear conclusions. Because this was a single-center study, there may also have been some selection bias and the sample size may not be large enough to represent the general situation. Therefore, we look forward to large-scale randomized controlled clinical trials to confirm the effects and proper usage of UTI in sepsis.
Acknowledgments
We thank Rebecca Tollefson, DVM, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study does not need ethical approval, because this study is a retrospective study in the real world, and the treatment of the patients conforms to the routine treatment of hospitals and relevant departments, and the data does not involve the privacy of patients.
References
- Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: a roadmap for future research. Lancet Infect Dis 2015;15:581-614. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Zhuo L, Salustri A, Kimata K. A physiological function of serum proteoglycan bikunin: the chondroitin sulfate moiety plays a central role. Glycoconj J 2002;19:241-7. [Crossref] [PubMed]
- Aosasa S, Ono S, Mochizuki H, et al. Mechanism of the inhibitory effect of protease inhibitor on tumor necrosis factor alpha production of monocytes. Shock 2001;15:101-5. [Crossref] [PubMed]
- Huang N, Wang F, Wang Y, et al. Ulinastatin improves survival of septic mice by suppressing inflammatory response and lymphocyte apoptosis. J Surg Res 2013;182:296-302. [Crossref] [PubMed]
- Linder A, Russell JA. An exciting candidate therapy for sepsis: ulinastatin, a urinary protease inhibitor. Intensive Care Med 2014;40:1164-7. [Crossref] [PubMed]
- Karnad DR, Bhadade R, Verma PK, et al. Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: a multicenter randomized controlled study. Intensive Care Med 2014;40:830-8. [Crossref] [PubMed]
- Xu Q, Yan Q, Chen S. Use of ulinastatin was associated with reduced mortality in critically ill patients with sepsis. J Thorac Dis 2019;11:1911-8. [Crossref] [PubMed]
- Xu Q, Yan Q, Chen S. Ulinastatin is effective in reducing mortality for critically ill patients with sepsis: a causal mediation analysis. Sci Rep 2018;8:14360. [Crossref] [PubMed]
- Cooperative Group of immunomodulatory Therapy of Sepsis, Lin HY. Clinical trial with a new immunomodulatory strategy: treatment of severe sepsis with Ulinastatin and Maipuxin. Zhonghua Yi Xue Za Zhi 2007;87:451-7.
- Chen Q, Hu C, Liu Y, et al. Safety and tolerability of high-dose ulinastatin after 2-hour intravenous infusion in adult healthy Chinese volunteers: A randomized, double-blind, placebo-controlled, ascending-dose study. PLoS One 2017;12:e0177425. [Crossref] [PubMed]
- Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med 2016;4:91. [Crossref] [PubMed]
- Zhang Z. Semi-parametric regression model for survival data: graphical visualization with R. Ann Transl Med 2016;4:461. [Crossref] [PubMed]
- Cao YZ, Tu YY, Chen X, et al. Protective effect of Ulinastatin against murine models of sepsis: inhibition of TNF-α and IL-6 and augmentation of IL-10 and IL-13. Exp Toxicol Pathol 2012;64:543-7. [Crossref] [PubMed]
- Li ST, Dai Q, Zhang SX, et al. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-κB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol Sin 2018;39:1294-304. [Crossref] [PubMed]
- Liu D, Yu Z, Yin J, et al. Effect of ulinastatin combined with thymosin alpha1 on sepsis: A systematic review and meta-analysis of Chinese and Indian patients. J Crit Care 2017;39:285-7. [Crossref] [PubMed]
Cite this article as: Zhu J, Liu Q, Cheng G, Zhang Z, Wang X. A retrospective study of the effectiveness of ulinastatin in the treatment of sepsis. J Emerg Crit Care Med 2020;4:10.