From cardiac output to peripheric perfusion: the perfusional pathway—a case report
Introduction
Rapid administration of intravenous fluid or a “fluid challenge” is one of the most common therapies in critically ill patients and represents the base of hemodynamic management in intensive care units (1). Fluid challenge was described as a way to evaluate the ability of the heart to take advantage of fluid load in presence of clinical signs and symptoms of insufficient circulation in order to improve tissue perfusion (2). However, liberal administration of fluids may lead to a positive fluid balance, which is an independent predictor of mortality in various categories of critically ill patients (3), in that it is associated with edema formation, organ congestion, dilution coagulopathy, hypotermia, dilution anemia, electrolyte imbalance (4). Therefore, individualized and goal-directed therapy based on prediction of fluid responsiveness by evaluation of change in stroke volume (SV) has largely developed in recent years. Furthermore, it was reported that blood flow to vital organs could increase in response to fluid administration in animal models even when SV changes are considered insignificant (no fluid responsiveness); hence SV variation (SVV) shouldn’t be used as a physiological parameter to evaluate improvement of regional perfusion (5). Tissue oxygenation depends on the rate of oxygen delivered to the tissue (DO2) and the rate of oxygen consumed by the tissue (VO2). The fraction of DO2 that diffuses from capillaries into tissues is defined as O2 extraction ratio (O2ER) and is expressed as a percentage of total DO2. When DO2 is reduced, O2ER increases enabling VO2 to be maintained. Below a critical point (DO2 crit) the increase in O2ER can no longer compensate for the fall in DO2. Therefore, VO2 declines in direct proportion to DO2 and becomes DO2 dependent. In our case we considered different hemodynamic parameters in sequence in order to identify fluid responsiveness, in terms of arterial blood pressure (BP) and cardiac output (CO) increase, and eventually find out if the improvement of BP would also be followed by an enhancement of tissutal perfusion.
Renal doppler resistive index (RDRI) is an ultrasound parameter, calculated with the following formula: (peak systolic velocity − end diastolic velocity)/peak systolic velocity, that involves the sampling of the renal interlobar or arcuate artery with pulsed wave Doppler. Hence it reflects the vascular resistance to flow of renal microcirculation (6). Therefore, it could be a tool to assess organ perfusion in critical illness (7) in that it may provide a very early detection of microcirculatory dysfunction even before biochemical and macro-hemodynamic changes.
Case presentation
A 75-year-oldman was admitted to the Intensive Care Unit of this hospital because of cardio-respiratory failure after a pulmonary lobectomy. He has a past medical history of diabetes mellitus type 2, Parkinson’s disease and senile dementia. During the following 3 days he developed a non-ST elevation myocardial infarction and a coronary angiogram was done. It revealed a critical stenosis of left anterior interventricular artery and left circumflex artery that couldn’t be treated with balloon angioplasty for the extension of coronary disease. Approximately 4 days after admission, his laboratory test showed an elevated leukocyte count (15.12/mm3), an haemoglobin level of 8.1 g/dL, a platelet count of 99×103 cells/µL, a creatinine level of 0.34 mg/dL; a total bilirubin level of 3.2 mg/dL, a procalcitonin level of 0.3 ng/mL and a Presepsin test of 2,269 ng/mL (Pathfast presepsin, Mitsubishi Chemical Europe, Düsseldorf, Germany). In addition, multiresistant Klebsiella pneumoniae were isolated in blood cultures, urinoculture and pharyngeal swab suggesting a condition of incipient sepsis that was treated with specific antibiotic therapy. He was sustained by mechanical ventilation with pressure support ventilation with the following parameters: support pressure14 cmH2O, positive end expiratory pressure (PEEP) 6 cmH2O, inspiratory oxygen fraction 40%.
His blood arterial gas analysis results were as follows: pH 7.55; PaCO2 40 mmHg; PaO2 70 mmHg; HCO3 33 mmol/L; lactate 1.4 mmol/L. As an alternative for mixed venous oxygen saturation (SvO2), we considered central venous oxygen saturation (SvcO2), according to the frequently reported evidence of correlation between SVO2 and SVCO2, that resulted 71.9% (8). The BP was 92/38 mmHg, mean arterial pressure (MAP) 56 mmHg, heart rate (HR) 74 bpm beats per minute, central venous pressure (CVP) 4 cmH2O. An echocardiography showed post-ischemic cardiomyopathy with an ejection fraction of 30% along with signs of hypertrophy and segmentary akinesia (mid-anterior and apical segment of left ventricle).
To assess a condition of fluid responsiveness, we performed a bedside cardiopulmonary ultrasound to measure SVV by analysing variations of velocity-time integral (VTI) of blood flow through aortic valve with respiratory changes in apical 5 chamber window with pulsed Doppler and inferior vena cava diameter variation (dIVCD) with motion-mode (M-mode) ultrasound. We obtained an SVV of 20% and no significant variation of the inferior vena cava diameter was noticed, probably due to PEEP. Aiming to evaluate the hemodynamic status we also measured the following parameters: SV 35 mL, CO 2.6 L/min, cardiac index (CI) 1.4 mL L/min/m2, systemic vascular resistance (SVR) 2,900 dyn·s·cm−5 and pulse pressure variation (PPV) by analysing arterial pressure waveform, that was 20%.
Then we considered vessel elastance (Eadyn), defined as the relationship between SVV and PVV that resulted approximately 1. Eadyn is reported to help foresee in which patients fluid challenge will increase BP and SV (9).
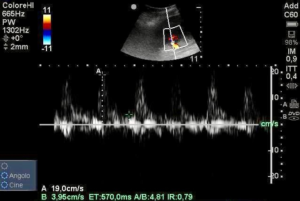
A pulsed wave Doppler ultrasound measurement of renal resistive index demonstrated a value of 0.79 (Figure 1) revealing hypoperfusion of renal microcirculation.
In order to evaluate kidney perfusion, we also evaluated the mean renal perfusion pressure (MPP), calculated as the difference between MAP and CVP, and the diastolic renal perfusion pressure (DPP), calculated as the difference between diastolic arterial pressure (DAP) and CVP. We find an MPP of 52 mmHg and a DPP of 34 mmHg.
Having considered all available data we supposed that the patient condition was due to a mixed shock, initial septic shock and cardiac shock but still had to determine which of the two had prevailed. Owing to suspected hypotension induced by septic shock and hypoperfusion showed by increased RDRI and low DPP, a fluid challenge was started immediately with an intravenous bolus of 500 mL of normal saline 0.9%, administered within 15 minutes.
Following fluid resuscitation, the vital signs were as follows: BP 138/55 mmHg, MAP 80 mmHg, HR 74 bpm and the CVP was 10 cmH2O. Blood gases values overlapped with the ones previously detected (pH 7.48, PaCO2 48 mmHg; PaO2 62 mmHg; HCO3 33.4 mmol/L; lactate 1.5 mmol/L) and no change was observed in SvcO2 that was 73%.
To compare the hemodynamic variables before and after volume challenge, we repeated the ultrasound measurement and we found a minimum increase of SV (41 mL, 14%) and CO (3.07 L/min, 15%). The fluid expansion resulted also in a decrease of SVV (14%) of 30%.
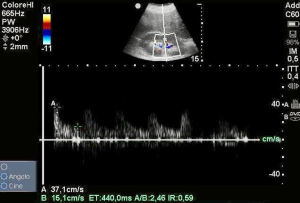
We repeated renal ultrasound that showed a normal value of RDRI of 0.59 (Figure 2), suggesting an enhancement of renal perfusion. Both MPP and DPP improved, indeed we collected a value of 80 and 50 mmHg respectively. The urine output remained unchanged before and after fluid challenge.
Discussion
Detection of parameters that could indicate an adequate therapeutic approach in conditions of septic shock has always been challenging. Rivers et al. identified different microcirculatory resuscitation targets to evaluate oxygen delivery to tissues. These parameters were: CVP, MAP, urine output, SvO2, ScvO2 and haematocrit (10). Greenwood and Orloski highlighted that these parameters, as well as CO, CI, lactate, central venous-arterial CO2 gradient, and capillary refill time are not fully reliable as microcirculatory resuscitation targets in patients with sepsis (11). The meaning of MAP itself has been questioned. Though a value of MAP >65 mmHg is recommended in patients with septic shock, the adequate MAP level to guarantee renal tissue perfusion is still under debate (12). Renal resistive index could be useful as a non-invasive and repeatable tool to assess changes in renal perfusion after a fluid load. This suggests that Doppler renal ultrasound may help to determine the optimal MAP for renal tissue perfusion. It may furthermore allow to compare changes in interlobal artery resistivity index with the ones in systemic hemodynamics parameters to predict modification of peripheral tissue perfusion after fluid resuscitation. Doppler ultrasonography and resistive index measurements may help determine in each patient the optimal MAP for renal blood flow and may be a relevant end-point to titrate the hemodynamic treatment in septic shock (12). Routine monitoring of hemodynamic parameters consists in the evaluation of pressures and flows in the macrocirculation with the purpose of managing fluids administration and vasoactive medication. Nevertheless, kidney vascularization is often disregarded by clinicians (13). RDRI measurement could provide important diagnostic and prognostic information and it is now gaining an ever-increasing role in the evaluation of AKI development and reversibility in critically ill patients (14,15). Moreover, Le Dorze et al. demonstrated that taking into consideration RDRI could support the optimization of renal perfusion in critical medical situations (7). RDRI depends on the interaction of multiple factors, which is why we can’t assume that it is a pure kidney-related index. Indeed, it is influenced by extra-renal factors such as pulse pressure, systemic vascular compliance, cardiac function and renal factors like renal capillary pressure and vascular resistance that reflect changing in kidney perfusion (6). Considering that splanchnic and renal perfusion could be defined as markers of global peripheral perfusion, RDRI may reflect global organ perfusion. It should be noted that RDRI is connected with perfusion pressure (16) therefore it could be considered as a sensitive predictor of initial phases of hypoperfusion caused by hypovolemia, hypotension, anaemia and cardiac shock. More interestingly, it has been reported that RDRI could predict tissutal hypoxia evidently earlier than any other hemodynamic parameter previously mentioned (17). In our case, while SvcO2 and lactate remained comparable before and after fluid challenge, we may observe that the only variable significantly changed after fluid load was RDRI whose value decreased to normality, hence RDRI is to be considered as essential in diagnostic procedure. We can suggest, consequently, that renal resistive index could be used to examine splanchnic and renal perfusion to identify the most appropriate CO or BP or fluid administration (in terms of amount of fluid) to sustain peripheral perfusion (18).
On the other hand, some authors found that volume expansion was not effective in decreasing renal resistive index in patients with septic shock (19), whereas others demonstrated the opposite (20).
The two differing results can be explained if we take into account what we observed in our case: a sequential evaluation of different parameters, starting from the source (CO) through peripheral vascular resistance to DO2 and VO2, is essential to appreciate global peripheral perfusion.
Moussa et al. also showed that modifications in RDRI after fluid resuscitation are more effective than changes in MAP and PPV to predict an increase in urine output (20). All the above is further confirmed by Schnell et al.; indeed they reported that significant variations of hemodynamic parameters, such as SV and CO, induced by fluid administration, did not translate into significant changes in RDRI (19).
In our case, having detected a condition of fluid responsiveness based on ultrasound measurement of SV and CO, we deduced that considering only SV and CO couldn’t guarantee the adequacy of our therapeutic choice and that only through the increase of RDRI we could identify and evaluate renal hypoperfusion determining the need of a fluid challenge. What is, furthermore, to be noted is that the improvement of renal and splanchnic perfusion was suggested by the decreasing of RDRI to normal value after fluid challenge, highlighting the reversibility of this index.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript.
References
- Cecconi M, Hofer C, Teboul JL, et al. Fluid challenges in intensive care: the FENICE study: A global inception cohort study. Intensive Care Med 2015;41:1529-37. [Crossref] [PubMed]
- Takala J. Volume responsive, but does the patient need volume? Intensive Care Med 2016;42:1461-3. [Crossref] [PubMed]
- Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006;34:344-53. [Crossref] [PubMed]
- Reuter DA, Chappell D, Perel A. The dark sides of fluid administration in the critically ill patient. Intensive Care Med 2018;44:1138-40. [Crossref] [PubMed]
- Brügger LE, Beldi G, Stalder M, et al. Postoperative splanchnic blood flow redistribution in response to fluid challenges in the presence and absence of endotoxemia in a porcine model. Shock 2012;37:116-21. [Crossref] [PubMed]
- Di Nicolò P, Granata A. Renal intraparenchymal resistive index: the ultrasonographic answer to many clinical questions. J Nephrol 2019;32:527-38. [Crossref] [PubMed]
- Le Dorze M, Bouglé A, Deruddre S, et al. Renal Doppler Ultrasound: A New Tool to Assess Renal Perfusion in Critical Illness. Shock 2012;37:360-5. [Crossref] [PubMed]
- Dueck MH, Klimek M, Appenrodt S, et al. Trends but Not Individual Values of Central Venous Oxygen Saturation Agree with Mixed Venous Oxygen Saturation during Varying Hemodynamic Conditions. Anesthesiology 2005;103:249-57. [Crossref] [PubMed]
- Monge García MI, Gil Cano A, Gracia Romero M. Dynamic arterial elastance to predict arterial pressure response to volume loading in preload-dependent patients. Crit Care 2011;15:R15. [Crossref] [PubMed]
- Rivers E, Nguyen B, Havstad S, et al. Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. N Engl J Med 2001;345:1368-77. [Crossref] [PubMed]
- Greenwood JC, Orloski CJ. End Points of Sepsis Resuscitation. Emerg Med Clin North Am 2017;35:93-107. [Crossref] [PubMed]
- Deruddre S, Cheisson G, Mazoit JX, et al. Renal arterial resistance in septic shock: effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensive Care Med 2007;33:1557-62. [Crossref] [PubMed]
- Rozemeijer S, Haitsma Mulier JL, Röttgering JG, et al. Renal resistive index: response to shock and its determinants in critically ill patients. Shock 2019;52:43-51. [Crossref] [PubMed]
- Darmon M, Schortgen F, Vargas F, et al. Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med 2011;37:68-76. [Crossref] [PubMed]
- Ninet S, Schnell D, Dewitte A, et al. Doppler-based renal resistive index for prediction of renal dysfunction reversibility: A systematic review and meta-analysis. J Crit Care 2015;30:629-5. [Crossref] [PubMed]
- Norris CS, Barnes RW. Renal artery flow velocity analysis: a sensitive measure of experimental and clinical renovascular resistance. J Surg Res 1984;36:230-6. [Crossref] [PubMed]
- Darmon M, Schortgen F, Leon R, et al. Impact of mild hypoxemia on renal function and renal resistive index during mechanical ventilation. Intensive Care Med 2009;35:1031-8. [Crossref] [PubMed]
- Ferré F, Marty P, Folcher C, et al. Effect of fluid challenge on renal resistive index after major orthopaedic surgery: A prospective observational study using Doppler ultrasonography. Anaesth Crit Care Pain Med 2019;38:147-52. [Crossref] [PubMed]
- Schnell D, Camous L, Guyomarc’h S, et al. Renal perfusion assessment by renal Doppler during fluid challenge in sepsis. Crit Care Med 2013;41:1214-20. [Crossref] [PubMed]
- Moussa MD, Scolletta S, Fagnoul D, et al. Effects of fluid administration on renal perfusion in critically ill patients. Critical Care 2015;19:250. [Crossref] [PubMed]
Cite this article as: Anile A, Ferrario S, Campanello L, Orban MA, Castiglione G. From cardiac output to peripheric perfusion: the perfusional pathway—a case report. J Emerg Crit Care Med 2020;4:2.