
Contemporary approaches to cardiopulmonary resuscitation: physiology-guided approaches
Introduction
The public health burden of out-of-hospital cardiac arrest (OHCA) is enormous, with over 350,000 cases yearly in the adult population of the United States (1). Despite a gradual decline in the incidence and absolute number of deaths, mortality remains unacceptably high at almost 90% for emergency medical service (EMS) treated OHCA. Although patients with a presenting rhythm of out-of-hospital ventricular fibrillation or pulseless ventricular tachycardia (VF/VT) have the highest survival rates compared with patients with non-shockable rhythms (2-7), more than 50% of patients with VF/VT OHCA are refractory to current treatment and never achieve return of spontaneous circulation (ROSC) or die before they are admitted to the hospital (8).
Cardiopulmonary resuscitation (CPR) is the cornerstone of cardiac arrest management in the acute setting. Since the first reported attempt by Claude Beck (9), and formation of the first guidelines for use by Kouwenhoven (10), chest compressions were the only means of cardiopulmonary support until defibrillation to induce ROSC. Since then, continuous and copious research efforts have shed some light into the pathophysiology of cardiac arrest. Autopsy studies and observation of survivors have uncovered the different etiologies of cardiac arrest and the most important challenges for successful recovery after ROSC. Application of this knowledge has helped guide management of cardiac arrest with novel devices, pharmacological agents and algorithms that aim to improve OHCA outcomes. In this review, we present the evidence-based physiology of cardiac arrest and associate it with established, ongoing and future approaches towards the management of cardiac arrest.
Blood flow during CPR
The mechanistic basis of CPR is an important concept that serves as an introduction to many of the other concepts discussed in this review. The fundamental driving force for blood flow during external chest compression is believed to be the pressure gradient that is generated between the thoracic cavity and the rest of the body. This gradient results in the concomitant increase of aortic pressure, carotid pressure and vital organ perfusion (11). Aortic pressure and right atrial pressure, which generate the perfusion pressure of the heart, possess a positive linear relationship with intrathoracic pressure that is unique to external compression and is preserved among species (12). Thus, blood flow occurs mainly due to the pressure gradients that are generated between the intrathoracic and extrathoracic vessels, as well as the release of blood into the aorta from the common conduit formed by left atrium and left ventricle during compression (13-20). In contrast to normal physiology, circulation during CPR partly depends on the thoracic pump mechanism, the increase in aortic pressure precedes aortic blood flow by approximately 40 milliseconds. Moreover, as the pressure is transmitted from the thorax to the aorta (rather than from the ventricle to the aorta) the aortic diameter decreases instead of increasing (21,22). Inside the thoracic cavity, blood flows from the vena cavae to the right ventricle during low intrathoracic pressure interval of CPR (decompression) and from the lungs to the left atrium during high intrathoracic pressure interval of CPR. However, despite the fact that the pulmonary valve closes in response to increases in intrathoracic pressure, negligible retrograde flow still occurs, mainly during the initiation of CPR and the opening of tricuspid valve (19,20).
The dependence of arterial blood flow on intrathoracic pressure variation and direct cardiac compression during the compression and decompression cycle has shaped the guidelines for good quality CPR. The main parameters that affect CPR quality have been deemed to be the duration of the compressive phase compared to the total duration of compression, also termed the ‘duty cycle’, the rate of compressions and the depth of compressions. The American Heart Association defines optimal CPR as a compression depth of ~5.5 cm, a compression rate between 100–120 bpm and a duty cycle of 50%, as coronary blood flow occurs mainly during the compression phase (19). These recommendations were confirmed in a recent observational study which assessed the impact of CPR quality on survival. Researchers identified a compression depth and rate of around 4.7 cm and 107 bpm, respectively as associated with the highest probability of survival for patients suffering from OHCA independent of the presenting rhythm (23).
The normal physiology discussed above has important physiologic limitations. While the object of CPR is to generate robust intrathoracic pressures with each compression, the rise in coronary perfusion pressures (CPPs) may be limited beyond a certain point due to the physical limitations of the thoracic structure. Further chest compressions produce a concomitant increase of both right atrial and aortic pressure during the compression and therefore maximal CPP can be only achieved during the decompression phase (24). Increased arterial tone, and increased blood flow generation increases CPP. Another critical parameter that limits the compression depth is the concomitant increase in intracranial pressure. This tends to neutralize the increase of systemic arterial pressure and minimize blood flow to the brain. Initially, it was demonstrated that the jugular vein valve effectively prevents retrograde flow and increase of intracranial pressure that would limit cerebral perfusion. However, it was soon discovered that jugular vein valves are dysfunctional in patients with chronically elevated central venous pressure. Moreover, the paravertebral venous and epidural plexuses permit transmission from high pressure compartments as they do not possess valves to inhibit backflow (25). In that notion, it was demonstrated that application of negative intrathoracic pressure during decompression phase of CPR resulted in a linear reduction of intracranial pressure and concomitant increase of cerebral perfusion pressure (26). Abdominal pressure during CPR may also be another limiting factor to successful resuscitation. Canine studies have indicated that CPR may create a pressure gradient between the abdominal and thoracic cavities. This is attributed to inversion of the diaphragm during CPR. This may consequently generate a negative pressure gradient that sequesters blood towards the abdomen and away from cerebral circulation (27).
Despite the established paradigm of the thoracic pump dominance during standard CPR, there is also evidence for a separate cardiac pump mechanism that might also affect circulation. Compression of the left ventricle may actually make the left ventricle more rigid, leading the left ventricle to merely become a passive conduit for blood flow (21). However, it has been noted that mitral valve mobility is affected and leaflets close in response to intrathoracic pressure elevations and open upon alleviation of this trigger (28). Moreover, both the right ventricle and left atrium respond to external compression, thus, a separate cardiac pump mechanism also exists and regulates blood flow during CPR (21).
As such, we currently accept that both mechanisms are involved in blood flow generation in different percentages based on the type of CPR. CPR methods with anteroporterior piston-like compressions favour a larger relative contribution of direct cardiac compression, while circumferential CPR methods, such as vest-CPR (29) and compression-band devices are thought to utilize the thoracic pump in a larger proportion.
Interventions targeting regulation of intrathoracic pressure
Understanding of the intrathoracic pressure effect on blood flow during CPR has been central to the development of experimental novel interventions and established clinical guidelines. The most important determinants of intrathoracic pressure during CPR are the interruptions during compression, ventilation, and the frequency and depth of compression itself. Edelson et al. previously revealed that interruptions in CPR and frequency of shallow compressions were inversely related to the probability of successful defibrillation (30). A post-hoc analysis of the prospective clinical trial PRIMED, which was performed to assess the efficacy of impedance threshold device (ITD), also confirmed the negative predictive value of pre- and peri-shock pauses in CPR on survival (31). In light of these findings, the contemporary resuscitation guidelines describe a preference for compression-only CPR in an attempt to minimize interruptions in high-quality CPR (32).
It has also become evident that rescuers tend to hyperventilate OHCA victims. This is a common finding even among well-trained EMS personnel (33). Hyperventilation is detrimental for survival as it creates an inverse relationship between CPP and endotracheal pressure. This has been demonstrated in porcine models treated with increased ventilation rates of either 20 or 30 breaths/minute. After treatment, the swine demonstrated significantly lower ROSC rates compared to swine treated with 12 breaths per minute. This may be due to the interruption of compressions to ventilate non-intubated swine or the additional positive intrathoracic pressure that is generated during ventilation that may consequently limit venous return during chest recoil after active compression. Moreover, when ventilation is applied, it was beneficial only at low tidal volumes and at a moderate respiratory rate, as a prospective study demonstrated that there were no survivors in case ventilation exceeded 18 breaths per minute (34).
As the effects of ventilation were becoming better understood, it was speculated that insertion of negative intrathoracic pressure during inspiration would have a positive effect on venous return and thus vital organ perfusion without any expense on tissue oxygenation (35,36). This rationale formed the basis for the creation of the ITD. The ITD works primarily by enhancing the vacuum effect that is observed during chest recoil to inhibit passive air entrance into the lungs (37,38). Thus, it can only be implemented in ventilated patients (39). Clinical trials have yielded mixed results. In the ROC-PRIMED study, ITD use did not demonstrate any superiority compared to conventional CPR (40). However, in another prospective study involving 2,470 patients, ITD use result in the marked increase in neurologically intact survival from 6% to 9% (2). These conflicting results have partially been traced back to confounders affecting the quality of resuscitative efforts (41). Thus, the role of the ITD in OHCA management remains undecided. Despite that, recent post hoc analyses evaluating the effect of the CPR quality on the effectiveness of the ITD have shown that when CPR is performed following current guidelines improved survival (23,42,43). The effect of negative intrathoracic pressure generation during CPR has been seen even without an ITD, since animals that gasp during cardiac arrest with a closed glottis have a significantly delayed onset of brain death (44).
The synchronization of ventilation with active compression is another method to optimize regulation of intrathoracic pressure during resuscitation. This has been postulated to improve cerebral blood flow and cerebral perfusion pressure (45). This hypothesis was confirmed when different modes of ventilation were assessed in porcine models of CPR. It was documented that synchronization of ventilation and chest compressions improved arterial pressures without sacrificing oxygenation (46). Unfortunately, the effects of very high intrathoracic pressures generated with this approach lead to lung injury and a significant rise of intracranial pressure and this approach was abandoned.
It has been demonstrated that during CPR with either conventional CPR or vest inflation, air expiration occurs in both inflation and deflation. The most plausible explanation is that due to airway collapse occurs due to the elevated intrathoracic pressure that occurs with CPR. This subsequently leads to air trapping in the lungs throughout the duration of compression or inflation. Air-trapping occurs independently of ventilation status (47). Understanding of this concept has led to the development of the pneumatic vest for CPR. This system allows the universal application of pressure along the thoracic wall and was associated with superior survival outcomes with lower CPR-associated trauma compared to conventional CPR (48). This application led to development of AutoPulse® (ZOLL Medical Corporation, Chelmsford, MA, USA) which, through the use of a band device, compresses the entire chest by delivering 80 compressions per minute (29,49). An automated mechanical piston for CPR delivery has been developed by Lund University, LUCAS® 3 (Lund University Cardiopulmonary Assist System). This system attempts to mimic the manual external compression design. The attempted head-to-head clinical trial did not demonstrate superiority of any of the two devices although it linked AutoPulse with a higher incidence of injuries during CPR (50). When compared to manual CPR, neither device has yielded superiority (51-57), probably due to the prolonged interruption that is required to deploy the device and initiate CPR (58). We now recognize that without a unified post-resuscitation management and cause-identification protocol, it is very difficult to have any prehospital intervention show a survival benefit.
Circulatory pump dysfunction: advanced cardiac life support (ACLS) strategies
The reasons for the inefficiency of left ventricle compression might lie in the effects of global cardiac ischemia that ensues after the arrest. At a cellular level, ischemia results in passive depolarization of the cardiomyocyte. Thus, in the initial phase, while excitability still occurs, action potentials have low amplitude and short duration (59). Depolarization occurs in response to constant activation of sodium channels that cause an inward movement of sodium in response to the local accumulation of lysophosphatidylcholine (60). Moreover, as ischemia progresses, cytosolic accumulation of calcium occurs and depolarization is further augmented (61). Intracellular calcium induces uncoupling of cells and loss of function in gap junctions (62). Furthermore, in response to ischemia, K+ effluxes from the cell and inactivates the voltage-gated sodium channels that are responsible for action potential generation, contributing to the changes observed upon the initial stage of depolarization (63). With myocardial ischemia, the refractory period initially shortens and then prolongs as ischemia progresses (64). Conversely, action potential duration decreases due to hypoxia-decreased calcium conductance. This is due to downregulation of inward calcium channels (65) and the opening of ATP dependent rectifying K+ channels (66). Action potential amplitude either decreases to a relatively stable value or demonstrates high beat to beat variability. Patch-clamp studies indicate that the variability is a result of altered calcium conductance in the early stage of ischemia (67). Eventually, ischemia leads to loss of contractility and mechanical failure (68). A summary of these changes is portrayed in Figure 1.
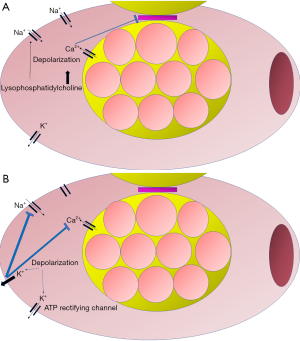
The loss of contractility also has detrimental effects on arterial tone. In acute heart failure patients, utilization of vasopressors not only did not decrease mortality but was associated with increased in-hospital deaths (69). For cardiac arrest, epinephrine has been the cornerstone of ACLS since 1960s. The first degree of uncertainty and understanding of limitations came from a 1994 study. The authors measured the cumulative dose of epinephrine and concluded that if it exceeded the critical limit of 15 milligrams, it was associated with a decreased rate of oxygen consumption and a concomitant lactate increase. Moreover, due to increased systemic vascular resistance, patients had lower cardiac index and worse oxygen delivery (70). Following publication of this study, multiple retrospective and prospective studies were conducted to evaluate the role of epinephrine. While epinephrine seemed to increased ROSC rate, there was no evidence of survival benefit (71-75). Moreover, retrospective evaluation of cardiac arrest cohorts indicated that epinephrine might be associated with lower rates of neurologically intact survival (76,77). In order to reconcile these conflicting results, researchers have tried to assess whether the time of epinephrine administration affects its efficacy. If epinephrine was provided close to the onset of OHCA, and also at the threshold of either 10 (78) or 20 (79) minutes, it could lead to higher neurologically intact survival compared to no intervention and also the other epinephrine groups. However, as previously demonstrated, the overall epinephrine population still had lower neurologically intact survival (80,81). There are also contradictory findings regarding the impact of presenting rhythm to the efficacy of epinephrine. For example, survival after OHCA with a shockable rhythm has been shown to be both higher (82) and lower (79) following epinephrine administration. Based on these reports, the American Heart Association has changed its recommendations. Standard dose epinephrine has been assigned a IIb level of evidence due to lack of demonstrated efficacy while high dose epinephrine is no longer recommended (83).
In contrast, potent vasodilators have been used in experimental models to determine whether vasodilation cam facilitate blood flow generated from the thoracic pump mechanism. Sodium nitroprusside is one such vasodilator that has been tested in porcine models of cardiac arrest. Compared to epinephrine, it has been associated with improved rates of ROSC and 4-hour survival (84). Sodium nitroprusside administration has also been associated with greater carotid blood flow and CPP (85). A prospective clinical trial is designed to assess the impact of sodium nitroprusside in the clinical setting.
Mechanical support
The recognition of the limited and largely stagnant survival outcomes with CPR and vasopressor-based ACLS approaches has led to an intense focus on the usage of mechanical support strategies as part of CPR. A variety of hemodynamic benefits have been posited for the use of mechanical support strategies—a reduction in preload, a reduction in afterload, limitation of ischemia-reperfusion injury, limitation of acidemia, and the provision of large volume hemodynamic support to mitigate the widespread multi-organ injury that ensues after the index cardiac arrest (86).
The earliest usage of mechanical support employed intra-aortic balloon pumps (IABPs). IABPs have the ability to be rapidly inserted in the cardiac catheterization laboratory to rapidly reduce afterload in patients with cardiogenic shock stemming from ventricular failure. The usage of IABP also increases the cardiac output by 0.5–1 L/minute, thus improving diastolic CPP (87). The IABP has traditionally been used to promote pulsatility in patients where there is no intrinsic pulsatility after resuscitation to prevent the occurrence of left ventricular thrombus and left ventricular ballooning (88,89). Despite a physiologically intuitive role in ameliorating cardiogenic shock in cardiac arrest, the routine usage of IABPs for the augmentation of cardiac output in cardiogenic shock have failed to show mortality benefit (90).
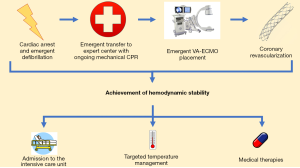
The use of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) evolved from the supposition that IABPs provided inadequate hemodynamic support in the midst of the post-cardiac arrest syndrome. With the rapid development of percutaneous VA-ECMO strategies, VA-ECMO can be inserted in the field, at the bedside, or in procedure suites to provide large volume hemodynamic support to aid resuscitative efforts (91). Importantly, cannulation for VA-ECMO can also be sought in a matter of minutes, making it possible to rapidly institute this strategy in the midst of ongoing chest compressions. The institution of VA-ECMO may allow for the reduction of transpulmonary flow while adequately providing perfusion to other vital organs. The Minnesota Resuscitation Consortium (MRC) has demonstrated a marked increase in neurologically favorable survival through the combined use of a bundled strategy that employs mechanical CPR, rapid transfer to the coronary catheterization laboratory, coronary revascularization, and hemodynamic support with percutaneous VA-ECMO (92,93). An illustrative summary of this approach is demonstrated in Figure 2. The routine use of VA-ECMO is, however, limited by the high level of expertise and advanced infrastructure required to employ this strategy routinely. Rising in afterload due to retrograde blood flow has been previously cited as a limitation of VA-ECMO usage (94). However, the physiologic effect of this increased afterload remains unclear in the setting of a dramatic decrease in left ventricular preload.
Outside of VA-ECMO, the use of percutaneous ventricular assist devices with or without VA-ECMO has also gained traction in the contemporary era (86). Percutaneous ventricular assist devices are inserted rapidly in the cardiac catheterization laboratory either at the time of the cardiac arrest or after resuscitation to facilitate hemodynamic support. In a study of four swine with induced ST-elevation myocardial infarction, the usage of percutaneous devices was associated with a reduction in infarct size when coupled with conventional therapies for myocardial infarction (95). This limited preclinical data has led to an increasing usage of percutaneous ventricular assist devices. There is also an increasing trend towards the concomitant usage of percutaneous ventricular assist devices alongside VA-ECMO (96). This is formed on the physiologic assumption that the usage of percutaneous VA-ECMO leads to an increase in afterload. There is little human data to confirm this assumption and it is unclear whether the rapid decline in preload offered by VA-ECMO can counteract the obvious rise in afterload in the cardiac end-diastolic pressure-volume relationship and lead to any increase in oxygen consumption in a state where cardiac function is severely suppressed. Thus, the true state of myocardial efficiency and whether the isolated usage of percutaneous ventricular assist devices without VA-ECMO in humans suffering from cardiac arrest leads to a survival benefit remains unclear. Percutaneous ventricular assist devices are prone to malpositioning. This may consequently lead to large volume hemolysis if this remains uncorrected, thus leaving patients prone to significant anemia and renal injury (97). Finally the use of peripheral ventricular assist devices (PVADs) does not provide oxygenation or gas exchange to support the severely compromised lung function in the majority of these patients. PVADs have a very poor performance during right ventricular failure and can only pump as much blood as it can be delivered to the LV via the transpulmonary flow during CPR. Those are significant limitations.
Neuroprotection
Protection of neurologic status and neuro-prognostication remain important challenges to successful resuscitation outcomes. Compared to the rest of organs, the brain has the lowest recovery rates and the longest time to recovery (98). The cornerstone of neuroprotection has been deemed to be high-quality CPR. A variety of additional in-hospital strategies have been trialed and implemented worldwide. Of them, targeted temperature management strategies, including therapeutic hypothermia, have risen to the fore in the last two decades. Targeted temperature management has been postulated to provide neuroprotection by inducing a hibernating state in metabolism to promote energy (adenosine triphosphate) conservation; altering the cellular stress response by. limiting inflammation and apoptosis; and enhancing angiogenesis, release of neural precursor cells, and increasing neuronal connectivity (99).
Initial randomized data suggest a highly favorable mortality and neurologic outcomes when hypothermia was used after ROSC (100). Since then, multiple high-quality trials have suggested that the neuroprotective effects of therapeutic hypothermia may be limited and/or similar with the maintenance of normothermia and prevention of febrile episodes (101). In particular, Nielsen et al. demonstrated in a large randomized controlled trial that the neurologic and survival benefit seen with hypothermia may in fact be similar with the avoidance of hyperthermia (102). Further randomized data has also suggested that some targeted temperature management strategies, such as the rapid infusion of cold saline, may even be associated with reduced survival (103). Current consensus guidelines in North America (104) and Europe (105) recommend the utilization of targeted temperature management to avoid febrile episodes while providing weaker recommendations for therapeutic hypothermia.
Cause-specific management
The one approach that has revolutionized treatment of cardiac arrest is the rapid identification and treatment of the cause. Since acute coronary syndromes due to coronary occlusion are the major cause of cardiac arrest due to shockable rhythms, early coronary angiography and urgent revascularization play a critical role in management (106). The central physiologic justification is that coronary artery disease incites the index cardiac arrest and the subsequent injury can be limited by urgent identification of coronary disease and its revascularization. Thus, immediate coronary angiography is rapidly becoming an important strategy in the peri-resuscitation and post-resuscitation care of patients with cardiac arrest.
Despite the emerging data to suggest the frequency of coronary artery disease and acute cardiac ischemia as the precipitating feature of cardiac arrest, there is still wide regional and international variation in the performance of emergent coronary angiography in patients after cardiac arrest (106). This may be due to the fact that consensus guidelines only offer clear recommendations for routine coronary angiography to be sought for resuscitated cardiac arrest after ST-elevation myocardial infarction, rather than all forms of cardiac arrest (107). In patients with non-shockable rhythms, there is a greater likelihood of an extracardiac etiology. However, there is still data to support early access to coronary angiography and revascularization as this population may still have significant underlying coronary artery disease as the inciting factor for the cardiac arrest in the absence of an overt history of coronary artery disease or electrocardiographic signs of coronary ischemia (108).
In the contemporary era, there is an increasing emphasis on seeking coronary angiography and revascularization in patients while CPR is ongoing. This is particularly pertinent to patients with shockable rhythms, given the improved survival with urgent coronary revascularization in this population (93). This has been made possible with automated CPR (e.g., LUCAS), as discussed above. This therapy remains limited by the infrastructure required to administer mechanical CPR, rapidly transfer patients to a center equipped with a coronary catheterization laboratory, rapidly pursue percutaneous coronary revascularization, and then institute the relevant post-cardiac arrest strategies. When all of the aforementioned can be sought, there is high-quality observational evidence that mortality and neurological outcomes can be dramatically improved (93,109). The Advanced REperfusion Strategies (ARREST) clinical trial (NCT03880565) is currently undergoing to prospectively assess the potential benefit of early entry into catheterization laboratory with ongoing CPR (110).
Coronary revascularization should be accompanied by the use of adjunctive medical therapies. Given that a significant number of patients suffer from a high burden of previously diagnosed and/or undiagnosed coronary artery disease, it is vital that the appropriate guideline-directed medical therapy (such as beta-blockade and statin therapy, as the systemic condition permits) is instituted at the earliest permissible opportunity to ensure plaque stabilization and prevent recurrences of malignant arrhythmias (111).
For non-ischemia associated cardiac arrest, both transthoracic echocardiography and point-of-care ultrasound are ubiquitous and perhaps the best studied modalities in the acute cardiac arrest setting. The diagnostic utility is self-evident, as readily reversible causes such as cardiac tamponade, hypovolemia, and pneumothorax may be identified via the use of the aforementioned techniques and rapidly alter the patient’s treatment trajectory. Peri-operative transesophageal echocardiography is also a mainstay in operative procedures that are associated with a high risk of hemodynamic compromise. Transesophageal echocardiography has also previously been shown to reliably identify the etiology of cardiac arrest in the operative setting (112). Intra-operative transesophageal echocardiography may provide information regarding the quality of cardiac compressions. Importantly, both transthoracic and transesophageal echocardiography may also identify the return of spontaneous ventricular contractions during resuscitative efforts before manual palpation of the pulses (113). Finally, echocardiography may offer important prognostic information to guide further resuscitative efforts.
The routine use of echocardiography and ultrasonography as part of the peri- and post-arrest care also has important limitations. The obtainment of diagnostic quality images is notoriously user-dependent with both echocardiography and ultrasonography. In a situation where there is significant movement of the patient, such as during CPR, transesophageal probe placement and obtainment of diagnostic utility ultrasound images may be particularly challenging. Finally, it is unclear how any of this information can alter the management of a patient and through that the outcomes. This area requires significant prospective research to identify the optimal role of imaging as part of resuscitation algorithms.
Conclusions
The physiology of CPR is particularly complicated and its understanding requires insights into the molecular, tissue and organ level of function. While physiology-guided resuscitative approaches have improved both survival and neurologic recovery, mortality rates remain high. This underscores the need for additional research that will lead to the discovery of either novel therapeutic tools or the more effective utilization of the currently available armamentarium. Until that time comes, the most important parameter for improving cardiac arrest survival is early optimization of perfusion by early identification of the cardiac arrest, bystander CPR, high quality professional CPR and, when available, extracorporeal cardiopulmonary resuscitation (ECPR) to normalize blood flow and offer an early “life line” in the absence of ROSC. The slogan “Push Hard, Push Fast, Do Not Stop” is still invaluable (114).
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139:e56-528. [Crossref] [PubMed]
- Aufderheide TP, Frascone RJ, Wayne MA, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet 2011;377:301-11. [Crossref] [PubMed]
- Wibrandt I, Norsted K, Schmidt H, et al. Predictors for outcome among cardiac arrest patients: the importance of initial cardiac arrest rhythm versus time to return of spontaneous circulation, a retrospective cohort study. BMC Emerg Med 2015;15:3. [Crossref] [PubMed]
- McNally B, Robb R, Mehta M, et al. Out-of-hospital cardiac arrest surveillance --- cardiac arrest registry to enhance survival (CARES), United States, October 1, 2005--December 31, 2010. MMWR Surveill Summ 2011;60:1-19. [PubMed]
- Stiell IG, Nichol G, Leroux BG, et al. Early versus later rhythm analysis in patients with out-of-hospital cardiac arrest. N Engl J Med 2011;365:787-97. [Crossref] [PubMed]
- Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 2008;300:1423-31. [Crossref] [PubMed]
- Daya MR, Schmicker RH, Zive DM, et al. Out-of-hospital cardiac arrest survival improving over time: results from the Resuscitation Outcomes Consortium (ROC). Resuscitation 2015;91:108-15. [Crossref] [PubMed]
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018;137:e67-492. [Crossref] [PubMed]
- Meyer JA. Claude Beck and cardiac resuscitation. Ann Thorac Surg 1988;45:103-5. [Crossref] [PubMed]
- Kouwenhoven WB, Jude JR, Knickerbocker GG. Closed-chest cardiac massage. JAMA 1960;173:1064-7. [Crossref] [PubMed]
- Chandra N, Rudikoff M, Weisfeldt ML. Simultaneous chest compression and ventilation at high airway pressure during cardiopulmonary resuscitation. Lancet 1980;1:175-8. [Crossref] [PubMed]
- Chandra N, Guerci A, Weisfeldt ML, et al. Contrasts between intrathoracic pressures during external chest compression and cardiac massage. Crit Care Med 1981;9:789-92. [Crossref] [PubMed]
- Babbs CF, Yannopoulos D. A dose-response curve for the negative bias pressure of an intrathoracic pressure regulator during CPR. Resuscitation 2006;71:365-8. [Crossref] [PubMed]
- Weisfeldt ML, Halperin HR. Cardiopulmonary resuscitation: beyond cardiac massage. Circulation 1986;74:443-8. [Crossref] [PubMed]
- Niemann JT, Rosborough JP, Ung S, et al. Coronary perfusion pressure during experimental cardiopulmonary resuscitation. Ann Emerg Med 1982;11:127-31. [Crossref] [PubMed]
- Babbs CF. Effects of an impedance threshold valve upon hemodynamics in Standard CPR: studies in a refined computational model. Resuscitation 2005;66:335-45. [Crossref] [PubMed]
- Niemann JT, Rosborough JP, Hausknecht M, et al. Pressure-synchronized cineangiography during experimental cardiopulmonary resuscitation. Circulation 1981;64:985-91. [Crossref] [PubMed]
- Criley JM, Niemann JT, Rosborough JP, et al. The heart is a conduit in CPR. Crit Care Med 1981;9:373-4. [Crossref] [PubMed]
- Cohen JM, Chandra N, Alderson PO, et al. Timing of pulmonary and systemic blood flow during intermittent high intrathoracic pressure cardiopulmonary resuscitation in the dog. Am J Cardiol 1982;49:1883-9. [Crossref] [PubMed]
- Werner JA, Greene HL, Janko CL, et al. Visualization of cardiac valve motion in man during external chest compression using two-dimensional echocardiography. Implications regarding the mechanism of blood flow. Circulation 1981;63:1417-21. [Crossref] [PubMed]
- Rich S, Wix HL, Shapiro EP. Clinical assessment of heart chamber size and valve motion during cardiopulmonary resuscitation by two-dimensional echocardiography. Am Heart J 1981;102:368-73. [Crossref] [PubMed]
- Guerci AD, Halperin HR, Beyar R, et al. Aortic diameter and pressure-flow sequence identify mechanism of blood flow during external chest compression in dogs. J Am Coll Cardiol 1989;14:790-8. [Crossref] [PubMed]
- Duval S, Pepe PE, Aufderheide TP, et al. Optimal combination of compression rate and depth during cardiopulmonary resuscitation for functionally favorable survival. JAMA Cardiol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Ditchey RV, Winkler JV, Rhodes CA. Relative lack of coronary blood flow during closed-chest resuscitation in dogs. Circulation 1982;66:297-302. [Crossref] [PubMed]
- Guerci AD, Shi AY, Levin H, et al. Transmission of intrathoracic pressure to the intracranial space during cardiopulmonary resuscitation in dogs. Circ Res 1985;56:20-30. [Crossref] [PubMed]
- Yannopoulos D, McKnite SH, Metzger A, et al. Intrathoracic pressure regulation for intracranial pressure management in normovolemic and hypovolemic pigs. Crit Care Med 2006;34:S495-500. [Crossref] [PubMed]
- Ducas J, Roussos C, Karsardis C, et al. Thoracicoabdominal mechanics during resuscitation maneuvers. Chest 1983;84:446-51. [Crossref] [PubMed]
- Halperin HR, Weiss JL, Guerci AD, et al. Cyclic elevation of intrathoracic pressure can close the mitral valve during cardiac arrest in dogs. Circulation 1988;78:754-60. [Crossref] [PubMed]
- Halperin HR, Tsitlik JE, Gelfand M, et al. A preliminary study of cardiopulmonary resuscitation by circumferential compression of the chest with use of a pneumatic vest. N Engl J Med 1993;329:762-8. [Crossref] [PubMed]
- Edelson DP, Abella BS, Kramer-Johansen J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation 2006;71:137-45. [Crossref] [PubMed]
- Cheskes S, Schmicker RH, Verbeek PR, et al. The impact of peri-shock pause on survival from out-of-hospital shockable cardiac arrest during the Resuscitation Outcomes Consortium PRIMED trial. Resuscitation 2014;85:336-42. [Crossref] [PubMed]
- Panchal AR, Berg KM, Kudenchuk PJ, et al. 2018 American Heart Association focused update on advanced cardiovascular life support use of antiarrhythmic drugs during and immediately after cardiac arrest: an update to the American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2018;138:e740-9. [Crossref] [PubMed]
- Aufderheide TP, Lurie KG. Death by hyperventilation: a common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med 2004;32:S345-51. [Crossref] [PubMed]
- Harmon MBA, van Meenen DMP, van der Veen ALIP, et al. Practice of mechanical ventilation in cardiac arrest patients and effects of targeted temperature management: a substudy of the targeted temperature management trial. Resuscitation 2018;129:29-36. [Crossref] [PubMed]
- Convertino VA, Ryan KL, Rickards CA, et al. Optimizing the respiratory pump: harnessing inspiratory resistance to treat systemic hypotension. Respir Care 2011;56:846-57. [Crossref] [PubMed]
- Segal N, Parquette B, Ziehr J, et al. Intrathoracic pressure regulation during cardiopulmonary resuscitation: a feasibility case-series. Resuscitation 2013;84:450-3. [Crossref] [PubMed]
- Yannopoulos D, Sigurdsson G, McKnite S, et al. Reducing ventilation frequency combined with an inspiratory impedance device improves CPR efficiency in swine model of cardiac arrest. Resuscitation 2004;61:75-82. [Crossref] [PubMed]
- Kwon Y, Debaty G, Puertas L, et al. Effect of regulating airway pressure on intrathoracic pressure and vital organ perfusion pressure during cardiopulmonary resuscitation: a non-randomized interventional cross-over study. Scand J Trauma Resusc Emerg Med 2015;23:83. [Crossref] [PubMed]
- Yannopoulos D, Aufderheide TP. Use of the impedance threshold device (ITD). Resuscitation 2007;75:192-3; author reply 193-4. [Crossref] [PubMed]
- Aufderheide TP, Nichol G, Rea TD, et al. A trial of an impedance threshold device in out-of-hospital cardiac arrest. N Engl J Med 2011;365:798-806. [Crossref] [PubMed]
- Wang CH, Tsai MS, Chang WT, et al. Active compression-decompression resuscitation and impedance threshold device for out-of-hospital cardiac arrest: a systematic review and metaanalysis of randomized controlled trials. Crit Care Med 2015;43:889-96. [Crossref] [PubMed]
- Yannopoulos D, Aufderheide TP, Abella BS, et al. Quality of CPR: an important effect modifier in cardiac arrest clinical outcomes and intervention effectiveness trials. Resuscitation 2015;94:106-13. [Crossref] [PubMed]
- Sugiyama A, Duval S, Nakamura Y, et al. Impedance threshold device combined with high-quality cardiopulmonary resuscitation improves survival with favorable neurological function after witnessed out-of-hospital cardiac arrest. Circ J 2016;80:2124-32. [Crossref] [PubMed]
- Bircher N, Safar P, Eshel G, et al. Cerebral and hemodynamic variables during cough-induced CPR in dogs. Crit Care Med 1982;10:104-7. [Crossref] [PubMed]
- Koehler RC, Chandra N, Guerci AD, et al. Augmentation of cerebral perfusion by simultaneous chest compression and lung inflation with abdominal binding after cardiac arrest in dogs. Circulation 1983;67:266-75. [Crossref] [PubMed]
- Kill C, Hahn O, Dietz F, et al. Mechanical ventilation during cardiopulmonary resuscitation with intermittent positive-pressure ventilation, bilevel ventilation, or chest compression synchronized ventilation in a pig model. Crit Care Med 2014;42:e89-95. [Crossref] [PubMed]
- Halperin HR, Brower R, Weisfeldt ML, et al. Air trapping in the lungs during cardiopulmonary resuscitation in dogs. A mechanism for generating changes in intrathoracic pressure. Circ Res 1989;65:946-54. [Crossref] [PubMed]
- Halperin HR, Guerci AD, Chandra N, et al. Vest inflation without simultaneous ventilation during cardiac arrest in dogs: improved survival from prolonged cardiopulmonary resuscitation. Circulation 1986;74:1407-15. [Crossref] [PubMed]
- Halperin H, Berger R, Chandra N, et al. Cardiopulmonary resuscitation with a hydraulic-pneumatic band. Crit Care Med 2000;28:N203-6.
- Koster RW, Beenen LF, van der Boom EB, et al. Safety of mechanical chest compression devices AutoPulse and LUCAS in cardiac arrest: a randomized clinical trial for non-inferiority. Eur Heart J 2017;38:3006-13. [Crossref] [PubMed]
- Rubertsson S, Lindgren E, Smekal D, et al. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in out-of-hospital cardiac arrest: the LINC randomized trial. JAMA 2014;311:53-61. [Crossref] [PubMed]
- Hallstrom A, Rea TD, Sayre MR, et al. Manual chest compression vs use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest: a randomized trial. JAMA 2006;295:2620-8. [Crossref] [PubMed]
- Zhu N, Chen Q, Jiang Z, et al. A meta-analysis of the resuscitative effects of mechanical and manual chest compression in out-of-hospital cardiac arrest patients. Crit Care 2019;23:100. [Crossref] [PubMed]
- Gates S, Lall R, Quinn T, et al. Prehospital randomised assessment of a mechanical compression device in out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised trial and economic evaluation. Health Technol Assess 2017;21:1-176. [Crossref] [PubMed]
- Perkins GD, Lall R, Quinn T, et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet 2015;385:947-55. [Crossref] [PubMed]
- Wik L, Olsen JA, Persse D, et al. Manual vs. integrated automatic load-distributing band CPR with equal survival after out of hospital cardiac arrest. The randomized CIRC trial. Resuscitation 2014;85:741-8. [Crossref] [PubMed]
- Savastano S, Baldi E, Palo A, et al. Load distributing band device for mechanical chest compressions: an Utstein-categories based analysis of survival to hospital discharge. Int J Cardiol 2019;287:81-5. [Crossref] [PubMed]
- Estock JL, Curinga HK, Li A, et al. Comparison of chest compression interruption times across 2 automated devices: a randomized, crossover simulation study. Am J Emerg Med 2016;34:57-62. [Crossref] [PubMed]
- Kléber AG, Janse MJ, van Capelle FJ, et al. Mechanism and time course of S-T and T-Q segment changes during acute regional myocardial ischemia in the pig heart determined by extracellular and intracellular recordings. Circ Res 1978;42:603-13. [Crossref] [PubMed]
- Undrovinas AI, Fleidervish IA, Makielski JC. Inward sodium current at resting potentials in single cardiac myocytes induced by the ischemic metabolite lysophosphatidylcholine. Circ Res 1992;71:1231-41. [Crossref] [PubMed]
- Clusin WT, Buchbinder M, Ellis AK, et al. Reduction of ischemic depolarization by the calcium channel blocker diltiazem. Correlation with improvement of ventricular conduction and early arrhythmias in the dog. Circ Res 1984;54:10-20. [Crossref] [PubMed]
- McCallister LP, Trapukdi S, Neely JR. Morphometric observations on the effects of ischemia in the isolated perfused rat heart. J Mol Cell Cardiol 1979;11:619-30. [Crossref] [PubMed]
- Wit AL. Basic electrophysiologic mechanisms of sudden cardiac death caused by acute myocardial ischemia and infarction. Card Electrophysiol Clin 2017;9:525-36. [Crossref] [PubMed]
- Downar E, Janse MJ, Durrer D. The effect of acute coronary artery occlusion on subepicardial transmembrane potentials in the intact porcine heart. Circulation 1977;56:217-24. [Crossref] [PubMed]
- Qian YW, Clusin WT, Lin SF, et al. Spatial heterogeneity of calcium transient alternans during the early phase of myocardial ischemia in the blood-perfused rabbit heart. Circulation 2001;104:2082-7. [Crossref] [PubMed]
- Isenberg G, Vereecke J, van der Heyden G, et al. The shortening of the action potential by DNP in guinea-pig ventricular myocytes is mediated by an increase of a time-independent K conductance. Pflugers Arch 1983;397:251-9. [Crossref] [PubMed]
- Isnberg G. Is potassium conductance of cardiac Purkinje fibres controlled by (Ca2+)? Nature 1975;253:273-4. [Crossref] [PubMed]
- Rovetto MJ, Whitmer JT, Neely JR. Comparison of the effects of anoxia and whole heart ischemia on carbohydrate utilization in isolated working rat hearts. Circ Res 1973;32:699-711. [Crossref] [PubMed]
- Mebazaa A, Motiejunaite J, Gayat E, et al. Long-term safety of intravenous cardiovascular agents in acute heart failure: results from the European Society of Cardiology Heart Failure Long-Term Registry. Eur J Heart Fail 2018;20:332-41. [Crossref] [PubMed]
- Rivers EP, Wortsman J, Rady MY, et al. The effect of the total cumulative epinephrine dose administered during human CPR on hemodynamic, oxygen transport, and utilization variables in the postresuscitation period. Chest 1994;106:1499-507. [Crossref] [PubMed]
- Holmberg M, Holmberg S, Herlitz J. Low chance of survival among patients requiring adrenaline (epinephrine) or intubation after out-of-hospital cardiac arrest in Sweden. Resuscitation 2002;54:37-45. [Crossref] [PubMed]
- Olasveengen TM, Sunde K, Brunborg C, et al. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA 2009;302:2222-9. [Crossref] [PubMed]
- Gueugniaud PY, Mols P, Goldstein P, et al. A comparison of repeated high doses and repeated standard doses of epinephrine for cardiac arrest outside the hospital. European Epinephrine Study Group. N Engl J Med 1998;339:1595-601. [Crossref] [PubMed]
- Herlitz J, Ekstrom L, Wennerblom B, et al. Adrenaline in out-of-hospital ventricular fibrillation. Does it make any difference? Resuscitation 1995;29:195-201. [Crossref] [PubMed]
- Jacobs IG, Finn JC, Jelinek GA, et al. Effect of adrenaline on survival in out-of-hospital cardiac arrest: a randomised double-blind placebo-controlled trial. Resuscitation 2011;82:1138-43. [Crossref] [PubMed]
- Olasveengen T.M, Wik L, Sunde K, et al. Outcome when adrenaline (epinephrine) was actually given vs. not given - post hoc analysis of a randomized clinical trial. Resuscitation 2012;83:327-32. [Crossref] [PubMed]
- Hagihara A, Hasegawa M, Abe T, et al. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA 2012;307:1161-8. [Crossref] [PubMed]
- Funada A, Goto Y, Tada H, et al. Effects of prehospital epinephrine administration on neurologically intact survival in bystander-witnessed out-of-hospital cardiac arrest patients with non-shockable rhythm depend on prehospital cardiopulmonary resuscitation duration required to hospital. Heart Vessels 2018;33:1525-33. [Crossref] [PubMed]
- Goto Y, Maeda T, Goto Y. Effects of prehospital epinephrine during out-of-hospital cardiac arrest with initial non-shockable rhythm: an observational cohort study. Crit Care 2013;17:R188. [Crossref] [PubMed]
- Nakahara S, Tomio J, Nishida M, et al. Association between timing of epinephrine administration and intact neurologic survival following out-of-hospital cardiac arrest in Japan: a population-based prospective observational study. Acad Emerg Med 2012;19:782-92. [Crossref] [PubMed]
- Perkins GD, Ji C, Deakin CD, et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med 2018;379:711-21. [Crossref] [PubMed]
- Koscik C, Pinawin A, McGovern H, et al. Rapid epinephrine administration improves early outcomes in out-of-hospital cardiac arrest. Resuscitation 2013;84:915-20. [Crossref] [PubMed]
- Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: adult advanced cardiovascular life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132:S444-64. [Crossref] [PubMed]
- Yannopoulos D, Bartos JA, George SA, et al. Sodium nitroprusside enhanced cardiopulmonary resuscitation improves short term survival in a porcine model of ischemic refractory ventricular fibrillation. Resuscitation 2017;110:6-11. [Crossref] [PubMed]
- Schultz J, Segal N, Kolbeck J, et al. Sodium nitroprusside enhanced cardiopulmonary resuscitation (SNPeCPR) improves vital organ perfusion pressures and carotid blood flow in a porcine model of cardiac arrest. Resuscitation 2012;83:374-77. [Crossref] [PubMed]
- Mandawat A, Rao SV. Percutaneous mechanical circulatory support devices in cardiogenic shock. Circ Cardiovasc Interv 2017. [Crossref] [PubMed]
- Emerman CL, Pinchak AC, Hagen JF, et al. Hemodynamic effects of the intra-aortic balloon pump during experimental cardiac arrest. Am J Emerg Med 1989;7:378-83. [Crossref] [PubMed]
- Petroni T, Harrois A, Amour J, et al. Intra-aortic balloon pump effects on macrocirculation and microcirculation in cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation*. Crit Care Med 2014;42:2075-82. [Crossref] [PubMed]
- Pappas G, Winter SD, Kopriva CJ, et al. Improvement of myocardial and other vital organ functions and metabolism with a simple method of pulsatile flow (IABP) during clinical cardiopulmonary bypass. Surgery 1975;77:34-44. [PubMed]
- Thiele H, Zeymer U, Thelemann N, et al. Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long-term 6-year outcome of the randomized IABP-SHOCK II trial. Circulation 2018. [Epub ahead of print]. [PubMed]
- Stub D, Bernard S, Pellegrino V, et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation 2015;86:88-94. [Crossref] [PubMed]
- Garcia S, Drexel T, Bekwelem W, et al. Early access to the cardiac catheterization laboratory for patients resuscitated from cardiac arrest due to a shockable rhythm: the Minnesota resuscitation consortium twin cities unified protocol. J Am Heart Assoc 2016. [Crossref] [PubMed]
- Yannopoulos D, Bartos JA, Raveendran G, et al. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol 2017;70:1109-17. [Crossref] [PubMed]
- Uriel N, Sayer G, Annamalai S, et al. Mechanical unloading in heart failure. J Am Coll Cardiol 2018;72:569-80. [Crossref] [PubMed]
- Kapur NK, Paruchuri V, Urbano-Morales JA, et al. Mechanically unloading the left ventricle before coronary reperfusion reduces left ventricular wall stress and myocardial infarct size. Circulation 2013;128:328-36. [Crossref] [PubMed]
- Pappalardo F, Schulte C, Pieri M, et al. Concomitant implantation of Impella((R)) on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 2017;19:404-12. [Crossref] [PubMed]
- Sibbald M, Dzavik V. Severe hemolysis associated with use of the Impella LP 2.5 mechanical assist device. Catheter Cardiovasc Interv 2012;80:840-4. [Crossref] [PubMed]
- Bartos JA, Carlson K, Carlson C, et al. Surviving refractory out-of-hospital ventricular fibrillation cardiac arrest: critical care and extracorporeal membrane oxygenation management. Resuscitation 2018;132:47-55. [Crossref] [PubMed]
- Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci 2012;13:267-78. [Crossref] [PubMed]
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557-63. [Crossref] [PubMed]
- Kalra R, Arora G, Patel N, et al. Targeted temperature management after cardiac arrest: systematic review and meta-analyses. Anesth Analg 2018;126:867-75. [Crossref] [PubMed]
- Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med 2013;369:2197-206. [Crossref] [PubMed]
- Bernard SA, Smith K, Finn J, et al. Induction of therapeutic hypothermia during out-of-hospital cardiac arrest using a rapid infusion of cold saline: the RINSE Trial (rapid infusion of cold normal saline). Circulation 2016;134:797-805. [Crossref] [PubMed]
- Callaway CW, Donnino MW, Fink EL, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132:S465-82. [Crossref] [PubMed]
- Nolan JP, Soar J, Cariou A, et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines for post-resuscitation care 2015: section 5 of the European Resuscitation Council guidelines for resuscitation 2015. Resuscitation 2015;95:202-22. [Crossref] [PubMed]
- Yannopoulos D, Bartos JA, Aufderheide TP, et al. The evolving role of the cardiac catheterization laboratory in the management of patients with out-of-hospital cardiac arrest: a scientific statement from the American Heart Association. Circulation 2019;139:e530-52. [Crossref] [PubMed]
- Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Socie. Eur Heart J 2018;39:119-77. [Crossref] [PubMed]
- Reynolds JC, Callaway CW, El Khoudary SR, et al. Coronary angiography predicts improved outcome following cardiac arrest: propensity-adjusted analysis. J Intensive Care Med 2009;24:179-86. [Crossref] [PubMed]
- Yannopoulos D, Bartos JA, Martin C, et al. Minnesota resuscitation consortium's advanced perfusion and reperfusion cardiac life support strategy for out-of-hospital refractory ventricular fibrillation. J Am Heart Assoc 2016. [Crossref] [PubMed]
- Advanced reperfusion strategies for refractory cardiac arrest (ARREST). Available online: https://clinicaltrials.gov/ct2/show/NCT03880565?id=NCT03880565&rank=1
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 2013;127:e362-425. [PubMed]
- van der Wouw PA, Koster RW, Delemarre BJ, et al. Diagnostic accuracy of transesophageal echocardiography during cardiopulmonary resuscitation. J Am Coll Cardiol 1997;30:780-3. [Crossref] [PubMed]
- Varriale P, Maldonado JM. Echocardiographic observations during in hospital cardiopulmonary resuscitation. Crit Care Med 1997;25:1717-20. [Crossref] [PubMed]
- Cone DC. Push hard, push fast, do not stop-optimal chest compression rate and depth. JAMA Cardiol 2019. [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Kosmopoulos M, Kalra R, Bartos JA, Raveendran G, Yannopoulos D. Contemporary approaches to cardiopulmonary resuscitation: physiology-guided approaches. J Emerg Crit Care Med 2020;4:19.


