
Intra-operative fluid restriction and post-operative pain management bundle associated with reduction in length of stay and opioid exposure in adolescent posterior spinal fusion patients
Introduction
Adolescent idiopathic scoliosis (AIS) is one of the most common forms of idiopathic scoliosis (1). Surgery has been shown to be a good therapeutic option for AIS. In particular, posterior spinal fusion surgery (PSF) is one of the most frequently performed orthopedic procedures that corrects AIS (2) and it has been shown to have better overall outcomes compared to other surgical approaches for AIS (3,4). In spite of the benefits of PSF, there are a variety of potential complications immediately post-surgery (5-13). PSF has been discovered to lead to significant blood loss (10-12) and significant pain post-operatively (13). These factors can lead to prolong return to function and increase length of stay in the Pediatric Intensive Care Unit (PICU). Several different strategies have been attempted individually to improve these risk with varying results.
An increase in total fluid administered is strongly associated with estimated blood loss (10), as well as poorer respiratory outcomes (14). As patients have greater blood loss they have increased blood transfusion risk (15). This is why it is important to come-up with strategies, both intra- and post-operatively, to reduce blood loss and tightly manage intravenous fluids.
Because PSF is a long invasive surgery causing massive tissue trauma and spinal nerve irritation, post-operative pain management is a real challenge. The mainstay medication for post-operative pain associated with PSF is opioids. However, opioids have multiple side-effects that limit dose escalation, and can lead to possible addiction long term (16-18). Additionally, opioids only target somatic pain and with PSF there is significant inflammatory and neuropathic pain not completely managed by opioids alone. To combat this, gabapentin has been tried as an adjuvant post-operatively and one recent study found that gabapentin decreased post-operative pain and opioid use in children (19). Another adjuvant tried to reduce opioid use while maintaining appropriate post-operative pain control in PSF is ketamine. This has however been shown to have various effects and the routine use of ketamine as an adjuvant in patients undergoing PSF still needs further research (20).
Given the current findings, our study aimed to see if a previously implemented standardized intra- and post-operative protocol using restricted intraoperative IV fluids and post-operative gabapentin, intravenous acetaminophen, intravenous ketorolac, and low dose ketamine would have an effect on various post-operative recovery time-dependent measures and opioid requirements. We predict that this standardized protocol would reduce time of foley removal, time to first mobilization, and length of stay. Our secondary hypothesis was that this standardized protocol would improve postoperative pain management and reduce opioid requirements. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jeccm-20-95) (21).
Methods
This study protocol was approved by the Naval Medical Center Portsmouth (NMCP) Institutional Review Board in compliance with all applicable federal regulations governing the protection of human subjects. Research data was derived from an approved IRB protocol: number NMCP.2019.0079. Written informed consent was not required by the Naval Medical Center Portsmouth IRB, as this data concerned historical de-identified patients. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study design
This was a single center, retrospective study of a cohort of patients ranging from 11 to 21 years with 0–2 comorbidities who required spinal surgical correction due to a Cobb angle greater than 50 degrees and/or a patient becoming symptomatic due to spinal curvature. Since this was a retrospective study, no follow-up of patients were obtained. Data was collected on April 4th to 31st 2020 from patients’ inpatient electronic medical records dated June 2011 to November 2019 at Naval Medical Center Portsmouth’s Pediatric Intensive Care Unit. Patients underwent PSF and recovered in the PICU. Eighteen patients were studied prior to the start of the PSF protocol and 26 patients were studied after protocol initiation retrospectively. Study size was based on the patient population obtained 4 years prior and after protocol initiation. This collection of data was determined to yield the greatest population size while decreasing confounders such as several different orthopedists performing spinal surgery correction. The protocol in this study was developed at NMCP by a group of Pediatric Intensivist, Pediatric Orthopedic Surgeons, and Anesthesiologist. Protocol consisted of restricting intravenous (IV) fluids to goal of no more than 1,500 mL intraoperatively, followed by post-operative pain management to include: ketamine infusion 3–5 mcg/kg/min which was turned off by afternoon of post-operative day 1, scheduled IV acetaminophen every 6 hours, scheduled gabapentin three times a day during post-operative day 1, scheduled ketorolac 0.5 mg/kg IV every 6 hours during post-operative day 1, hydromorphone or morphine PCA without basal rate which was discontinued on post-operative day 2, and dantrolene (0.5 mg/kg IV every 12 hours, with maximum of 20 mg/dose) or diazepam (0.05 to 0.2 mg/kg) for muscle spasticity daily post-operatively. Each patient was given isotonic maintenance IV fluids post-operatively. The Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) was performed with vital checks by the patient’s bedside nurse and was averaged per shift to evaluate patient’s subjective pain for this study (22). All opioid medication given daily for the first 3 days post-operative were standardized to morphine equivalents.
During surgery, those on protocol received the following: in pre-operative bay, patients received 1 dose of gabapentin 5 mg/kg/dose (maximum of 300 mg) orally. Midazolam was given as a pre-medication and mask induction was started. IV placement and intubation was then performed. Rocuronium 0.5 mg/kg was then given and second IV was obtained. Propofol was used as anesthetic medication. Patient was then flipped from a supine to a prone position. Total intravenous anesthesia (TIVA) started to obtain baseline motor evoked potentials (MEPs). Bolus medications to include tranexamic acid 10–30 mg/kg over 15 min, ketamine 0.5–2 mg/kg, methadone 0.1 mg/kg, and fentanyl 2–5 mcg/kg were given just before incision. During spinal dissection 0.5 mg/kg of rocuronium was given and then sevoflurane gas. Sevoflurane gas was then turned off at end of dissection upon wound closure, then propofol and remifentanil were stopped. Re-dose of methadone 0.1 mg/kg and bolus of diazepam 0.05–0.2 mg/kg were then given. Nitrous oxide at 50% was then turned on, and at end of surgery patient was placed from prone to supine. The patient was then extubated and transported to the PICU.
Statistics
All data was reviewed using standard descriptive univariate statistics, and evaluated for normality using the Kolmogorov-Smirnov test. Analysis was conducted using Stata 15, StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC. Comparisons between groups were conducted with either the Mann-Whitney test, or t-tests when appropriate. Mann-Whitney U nonparametric test between independent groups was performed to compare weight, BMI, LOS, foley days, time to first out of bed, and morphine equivalent totals per day between the two main groups in this study (pre-protocol and protocol group). T-test was performed for age, degree of curvature, number of vertebrae fused, length of procedure, operative fluid requirements, daily post-operative fluid requirements, EBL, and subjective pain. Pearson Correlation statistics were performed to study possible correlations between independent variables and length of stay. Statistical significance was considered when two-tailed P<0.05. All patient daily electronic patient records were reviewed for adverse outcomes to include: hypotension requiring intervention, need for continued intubation, requirement of respiratory support, blood products required (red blood cell transfusion and fresh frozen plasma), hypocalcemia needing repletion, and SIADH. All adverse outcomes were compared between the two groups using Fisher’s exact test. Data collection bias was addressed by the researcher who was collecting data was unaware of the protocol each patient was in until analysis of collected data was performed.
Results
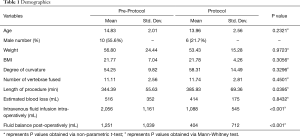
Forty-four patients who underwent PSF in our institution were retrospectively studied. Out of these patients, twenty-six were studied who underwent our PSF protocol. In our study, the demographics were similar between the groups except the protocol group had statistically significant longer surgical time (P=0.0395, Table 1). As planned, the protocol group had over 1,127 mL less of total intravenous fluids (pre-protocol 2,194 mL versus protocol 1,067, P≤0.001) and had almost 1,000 mL less of total fluid balance after surgery (pre-protocol 1,390 mL versus protocol 396 mL; P=0.008) compared to the pre-protocol group. Both groups had similar estimate blood loss (pre-protocol 516 mL versus protocol 414 mL; P=0.8432), but the protocol group were given less returned cell-saver blood (pre-protocol 165 mL versus protocol 96 mL; P=0.0199). When looking at both groups from a fluid management stand point, there was no difference in the post-operative blood product requirement (P≥0.999, Table 2), signs of fluid overload (P≥0.999, Table 2), and daily post-operative fluid intake requirements (day 1 pre-protocol 2,125 mL versus protocol 2,222 mL, P≥0.999; day 2 pre-protocol 2,567 mL versus protocol 3,164 mL, P≥0.999; and day 3 pre-protocol 2,089 mL versus protocol 1,934 mL, P≥0.999).
Full table

Full table
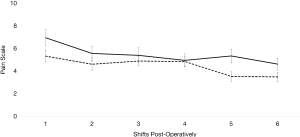
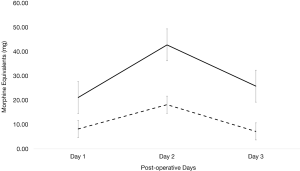
For subjective pain, measured by CHEOPS scoring, there was no significant difference between each group for the first 6 shifts post-operatively (on day 1 shift 1 P=0.0528, shift 2 P=0.3341; day 2 shift 1 P=0.6559, shift 2 P=0.9519; and day 3 shift 1 P=0.0301, shift 2 P=0.141 post operatively; Figure 1) except on day 3 shift 1 (mean pre-protocol 5 versus mean protocol 3.5, P=0.0301). Even though subjective pain was overall similar between the two groups, the amount of morphine equivalent pain medication required between each group greatly differed. Each day post-operatively, the protocol group required significantly less morphine equivalents (day 1 P≤0.001, day 2 P≤0.001, and day 3 P≤0.001). On day 1, pre-protocol group required 21.16 mg of morphine equivalents compared to 8.37 mg of morphine equivalents (Figure 2). On day 2, pre-protocol group required 42.82 mg of morphine equivalents compared to 18.45 mg of morphine equivalents (Figure 2). Finally, on day 3, pre-protocol group required 25.85 mg of morphine equivalents compared to 7.17 mg of morphine equivalents (Figure 2).
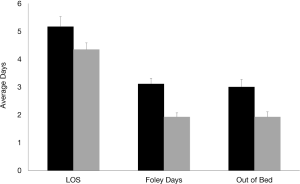
When analyzing time-dependent variables between both groups, it was found that the protocol group had significantly less LOS (pre-protocol 5.2 days versus protocol 4.3 days, P=0.0131), days of foley insertion (pre-protocol 3.1 days versus protocol 1.8 days, P≤0.001), and time to first get out of bed post-operatively (pre-protocol 3.2 days versus protocol 1.8 days, P=0.0002) (Figure 3).
Given that the pre-protocol group required significantly more opioids and had a significantly longer LOS, Pearson correlation studies were performed. It was found that increase of morphine equivalent total was positively correlated to increase length of stay (P=0.0031) (Table 3).

Full table
There was no significant difference in adverse outcomes between pre-protocol and protocol group (Table 2). All hypotension experienced was transient and required only a normal saline bolus (20 mL/kg) to resolve.
Discussion
In our single center retrospective study, we found a clinically significant reduction of opioid medication while maintaining appropriate pain control (Figures 1,2). This is most likely due to the use of non-opioid pain medications used such as ketamine infusion, gabapentin, IV acetaminophen, and IV ketorolac. To our knowledge, our study is the first to investigate this combination of post-operative pain management in pediatric PSF surgeries.
There has been mix data of the effectiveness of gabapentin to manage pain in pediatric PSF (23,24). However, it appears that the maximum benefit from gabapentin is within 48–72 hours post-operatively (19,24). Our results show significance at 24 hours, however there is a greater difference in opioid requirements at 48–72 hours post-operative. This agrees with the findings of Trzcinski et al. (19).
Ketamine use in spinal fusion surgery has also lead to mixed results, however it appears that lower dose ketamine infusions decrease cumulative morphine consumption by at least 20% in a previous study by Minoshima et al. (25). Based on our results, we agree with Minoshima et al. We too, saw a significant decrease in daily cumulative morphine consumption of about 56-72% decrease between groups. The reason our consumption difference was so much more then Minoshima et al.’s study was because of the cumulative effects of our non-opioid pain management bundle.
In regards to acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) use in PSF, our study agrees with the limited data that scheduled acetaminophen and NSAIDs reduces opioid requirements and improves analgesia (26). A concern that has started to be seen in the literature is IV acetaminophen leading to transient hypotension that leads to negative outcomes (27). But, these studies were on critical ill non-cardiovascular healthy children, which this did not apply to our patient population. We did see some hypotension events, but these patients only required a normal saline bolus to resolve (Table 2). This data agrees to the safety of acetaminophen as our previously published work (28). One concern with ketorolac is its potential to increase postoperative blood loss, however there was no significant increase in blood product requirements in our protocol group versus pre-protocol group (Table 2). Based on our significant results and previous data, there appears to be an accumulative decreased opioid consumption effect of combining scheduled gabapentin, IV acetaminophen, and ketorolac and ketamine infusion.
With the reduction of opioid consumption in our protocol population and no increase in adverse outcomes (Table 3), we saw that the protocol group also had significant reduction in time-dependent recovery variables (Figure 3). This quicker recovery time and reduced LOS is most likely multi-factorial. By reducing opioid daily amounts, reduces opioid related side effects that can lead to increase LOS (29). Our results agree with Olbrecht et al., who found that IV acetaminophen in PSF significantly reduced LOS (30). This association of reduced recovery time and LOS was further strengthen by our study finding that increase of morphine equivalent total was positively correlated to increase length of stay (P=0.0031; Table 3).
Another novelty of our study was intra-operative fluid restriction in our protocol group. Other studies have shown that increase intra-operative fluids can lead to increase estimated blood loss and negative respiratory outcomes (10,21). In our study, we saw a trend of the protocol group that was fluid restricted had about 51 mL estimated blood loss less compared to those that were not fluid restricted but this did not reach significance (P=0.8432). There was no difference in their blood product requirements post-operatively (P≥0.999) and no difference in their post-operative fluid requirements (day 1 P≥0.999, day 2 P≥0.999, and day 3 P≥0.999; Table 3). There was no significant difference between respiratory support in our patient group population (P≥0.999). Thus, it is unclear how much intra-operative fluid restriction plays a role at reducing recovery time and decreasing blood product requirements.
A limitation to this study is that it is a retrospective study, and thus only associations based on our findings can be made. Also, because this study was retrospective, we did not have the ability to separate groups into fluid restrictive only group, post-operative non-opioid based management, combined group, and current practice group. Thus, based on our results, we suggest further larger scale prospective studies that separate patients into the above groups to investigate the exact benefits of our combined PSF bundle compared to current measures. Another limitation is our patient population size. Given the length of surgery and overall occurrence in our hospital, in order to increase our study’s population size, this study would take significantly longer to complete. This would limit real time results to further medical care in this population. When interpreting our data, these conclusions can only be made to our specific population of overall low comorbid 11 to 21 years old patients with significant symptomatic or severe spinal curvature scoliosis.
Conclusions
The intra-operative fluid restriction and post-operative bundle of IV medication to manage inflammation and neuropathic pain is associated with clinically significant reduction of recovery time and opioid medication requirements after PSF surgery. This improvement was seen with no additional adverse outcomes. Thus, based on our specific population and retrospective data, we recommend reduction of intra-operative fluids and use of our post-operative pain management bundle for future large scale prospective studies to prove causation and provide greater generalizability.
Acknowledgments
We like to thank all Pediatric Intensivists and Pediatric Orthopedic Surgeons involved in patient care. We would also like to thank Andrea McGlynn with her help and guidance with statistics used in this study.
Funding: None.
Footnote
Reporting Checklist: The authors of this manuscript have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jeccm-20-95
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jeccm-20-95
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jeccm-20-95). The authors have no conflicts of interest to declare. The authors are military service members and employees of the U.S. Government. This work was prepared as part of our official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties. The views expressed in this manuscript reflect the results of research conducted by the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study protocol was approved by the Naval Medical Center Portsmouth (NMCP) Institutional Review Board in compliance with all applicable federal regulations governing the protection of human subjects. Research data was derived from an approved IRB protocol: number NMCP.2019.0079. Written informed consent was not required by the Naval Medical Center Portsmouth IRB, as this data concerned historical de-identified patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burton MS. Diagnosis and treatment of adolescent idiopathic scoliosis. Pediatr Ann 2013;42:224-8. [Crossref] [PubMed]
- Bourget-Murray J, Ferri-de-Barros F. Reinventing the wheel in scoliosis surgery: effective strategies for safely improving efficiency. Can J Surg 2019;62:7-8. [Crossref] [PubMed]
- Geck MJ, Rinella A, Hawthorne D, et al. Comparison of surgical treatment in adolescent idiopathic scoliosis: anterior dual rod versus posterior pedicle fixation surgery: a comparison of two practices. Spine 2009;34:1942-51. [Crossref] [PubMed]
- Huitema G, Willems PC, van Rhijn L, et al. Anterior versus posterior spinal correction and fusion for adolescent idiopathic scoliosis. Cochrane Database of Systematic Reviews 2014.CD011280. [Crossref]
- Bosch P, Kenkre TS, Londino JA, et al. Coagulation Profile of Patients with Adolescent Idiopathic Scoliosis Undergoing Posterior Spinal Fusion. J Bone Joint Surg Am 2016;98:e88. [Crossref] [PubMed]
- Baker CE, Marvi T, Austin TM, et al. Dilutional coagulopathy in pediatric scoliosis surgery: A single center report. Paediatr Anaesth 2018;28:974-81. [Crossref] [PubMed]
- Hassmann GC, Keim HA. Disseminated intravascular coagulation (DIC) in orthopedic surgery. Case report and review of literature. Clin Orthop 1974.118-32. [Crossref] [PubMed]
- Kannan S, Meert KL, Mooney JF, et al. Bleeding and coagulation changes during spinal fusion surgery: A comparison of neuromuscular and idiopathic scoliosis patients. Pediatr Crit Care Med 2002;3:364-9. [Crossref] [PubMed]
- Stanitski CL, Whittlesey G, Thompson I, et al. Clotting parameters in patients with adolescent idiopathic scoliosis undergoing posterior spinal fusion and instrumentation. J Pediatr Orthop B 1998;7:132-4. [Crossref] [PubMed]
- Shapiro F, Sethna N. Blood loss in pediatric spine surgery. Eur Spine J 2004;13 Suppl 1:S6-17. [Crossref] [PubMed]
- Nuttall GA, Horlocker TT, Santrach PJ, et al. Predictors of blood transfusions in spinal instrumentation and fusion surgery. Spine 2000;25:596-601. [Crossref] [PubMed]
- Meert KL, Kannan S, Mooney JF. Predictors of red cell transfusion in children and adolescents undergoing spinal fusion surgery. Spine 2002;27:2137-42. [Crossref] [PubMed]
- Sheffer BW, Kelly DM, Rhodes LN, et al. Perioperative pain management in pediatric spine surgery. Orthop Clin North Am 2017;48:481-6. [Crossref] [PubMed]
- Niescery J, Huhmann N, Dasch B, et al. Effects of liberal vs. conventional volume regimen on pulmonary function in posterior scoliosis surgery. Middle East J Anaesthesiol 2013;22:165-71. [PubMed]
- Nugent M, Tarrant RC, Queally JM, et al. Influence of curve magnitude and other variables on operative time, blood loss and transfusion requirements in adolescent idiopathic scoliosis. Ir J Med Sci 2016;185:513-20. [Crossref] [PubMed]
- Gil JA, Gunaseelan V, DeFroda SF, et al. Risk of Prolonged Opioid Use Among Opioid-Naïve Patients After Common Shoulder Arthroscopy Procedures. Am J Sports Med 2019;47:1043-50. [Crossref] [PubMed]
- Stark N, Kerr S, Stevens J. Prevalence and predictors of persistent post-surgical opioid use: a prospective observational cohort study. Anaesth Intensive Care 2017;45:700-6. [Crossref] [PubMed]
- Yang S, Werner BC. Risk factors for prolonged postoperative opioid use after spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop 2019;39:500-4. [Crossref] [PubMed]
- Trzcinski S, Rosenberg RE, Vasquez Montes D, et al. Use of gabapentin in posterior spinal fusion is associated with decreased postoperative pain and opioid use in children and adolescents. Clin Spine Surg 2019;32:210-4. [Crossref] [PubMed]
- Seki H, Ideno S, Ishihara T, et al. Postoperative pain management in patients undergoing posterior spinal fusion for adolescent idiopathic scoliosis: a narrative review. Scoliosis Spinal Disord 2018;13:17. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. [Crossref] [PubMed]
- McGrath PJ, Johnson G, Goodman JT, et al. CHEOPS: a behavioral scale for rating postoperative pain in children. In: Fields HL, Dubner R, Cervero F. editors. Advances in Pain Research and Therapy. New York: Raven Press, 1985:395-402.
- Mayell A, Srinivasan I, Campbell F, et al. Analgesic effects of gabapentin after scoliosis surgery in children: a randomized controlled trial. Paediatr Anaesth 2014;24:1239-44. [Crossref] [PubMed]
- Rusy LM, Hainsworth KR, Nelson TJ, et al. Gabapentin use in pediatric spinal fusion patients: a randomized, double-blind, controlled trial. Anesth Analg 2010;110:1393-8. [Crossref] [PubMed]
- Minoshima R, Kosugi S, Nishimura D, et al. Intra- and postoperative low-dose ketamine for adolescent idiopathic scoliosis surgery: a randomized controlled trial. Acta Anaesthesiol Scand 2015;59:1260-8. [Crossref] [PubMed]
- Wong I, St John-Green C, Walker SM. Opioid-sparing effects of perioperative paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) in children. Paediatr Anaesth 2013;23:475-95. [Crossref] [PubMed]
- Achuff BJ, Moffett BS, Acosta S, et al. Hypotensive Response to IV Acetaminophen in Pediatric Cardiac Patients. Pediatr Crit Care Med 2019;20:527-33. [Crossref] [PubMed]
- Mari D, Biswas A. Transient hypotension from intravenous acetaminophen in adolescent post-operative posterior spinal fusion surgery. J Emerg Crit Care Med 2020;4:15. [Crossref]
- Pizzi LT, Toner R, Foley K, et al. Relationship between potential opioid-related adverse effects and hospital length of stay in patients receiving opioids after orthopedic surgery. Pharmacotherapy 2012;32:502-14. [Crossref] [PubMed]
- Olbrecht VA, Ding L, Spruance K, et al. Intravenous acetaminophen reduces length of stay via mediation of postoperative opioid consumption after posterior spinal fusion in a pediatric cohort. Clin J Pain 2018;34:593-9. [Crossref] [PubMed]
Cite this article as: Mari DC, Biswas AK. Intra-operative fluid restriction and post-operative pain management bundle associated with reduction in length of stay and opioid exposure in adolescent posterior spinal fusion patients. J Emerg Crit Care Med 2021;5:2.


