Acute lung injury following gadolinium contrast: a case report
Introduction
For over 30 years gadolinium-based agents have been the most commonly used forms of intravenous contrast for magnetic resonance imaging (MRI) (1). Gadobutrol is a second-generation macrocyclic non-ionic contrast with high signal-to-dose ratio that was first introduced in 1998. In the decades since its introduction, gadobutrol has consistently demonstrated an excellent safety profile, with generally mild and self-limited side effects including nausea, headache, and dizziness (2). To our knowledge, only five prior cases have been described in the literature of non-cardiogenic pulmonary edema related to administration of gadolinium-based contrast, none of which were in children (3-6). Here, we describe the case of a 10-year old boy admitted to our pediatric intensive care unit (PICU) with methicillin-sensitive staphylococcus aureus sepsis who developed acute non-cardiogenic pulmonary edema after administration of gadobutrol. We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jeccm-20-117).
Case presentation
We report a previously healthy ten-year old boy was admitted to our PICU with knee pain and sepsis. He had experienced unilateral right knee pain, fever, and malaise for several days and had high fever, lethargy, altered mental status, and anuria 24 hours prior to admission. His exam was notable for poorly perfused extremities, a S3 gallop, bilateral rales, and a diffuse papular and pustular rash with some desquamation involving the extremities and face. The white blood cell count was 6,000/mL with 8% bands, pH 7.29, lactate 5.7, AST 321, ALT 159, BUN 72, and Cr 4.5 (Table 1). A knee joint aspirate demonstrated 109k WBC’s/mL, with 91% polymorphonuclear cells. A chest radiograph (CXR) revealed multifocal lung opacities, pulmonary edema, and bilateral pleural effusions. An echocardiogram demonstrated globally depressed biventricular function (left ventricular ejection fraction of 46%) and poor ventricular compliance and relaxation.

Full table
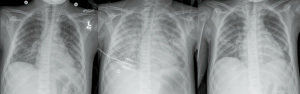
The patient was intubated for respiratory failure and was administered intravenous fluids, norepinephrine, and corticosteroids for hypotension and broad-spectrum antibiotic therapy including ceftriaxone, vancomycin, doxycycline, and clindamycin. By hospital day (HD) 3, blood and knee joint fluid cultures grew methicillin-sensitive staph aureus, and antibiotic coverage was narrowed to nafcillin and rifampin. On hospital day 5, he developed bilateral pleural effusions requiring chest tube drainage. Over the next two weeks, the patient’s cardiac function returned to normal. He was extubated to CPAP on HD14 and weaned to room air on HD15. The chest tube was removed on HD15. At this time norepinephrine had been discontinued, his renal function indices were normal, and no there were no additional positive blood cultures.
On HD 20 the patient developed progressive right-sided weakness in his upper and lower extremities, fecal and urinary incontinence, hypertension requiring a nicardipine infusion, and generalized tonic-clonic seizures that resolved with intravenous lorazepam and levetiracetam. Head computed tomography ruled out intracerebral hemorrhage. On HD22, the patient underwent sedation with gadolinium contrast and magnetic resonance imaging (MRI) that demonstrated findings consistent with posterior-reversible encephalopathy syndrome (PRES). An echocardiogram two hours prior to gadolinium administration demonstrated normal cardiac systolic and diastolic function and an ejection fraction of 68%. The BUN and Cr that day were 5 and 0.65, respectively, and fluid balance for the entire hospitalization was nearly even (Table 1). During the MRI, the patient received 0.23 mL/kg (11.2 mL) of gadoterate meglumine, 38.5 mg/kg (1,867 mg) propofol over two hours, and a total of 16 mL/kg of intravenous fluids. Arterial oxygen saturation in room air was greater than 96% throughout the procedure and after emerging from sedation.
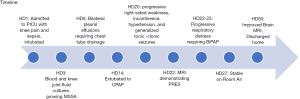
Three hours following gadolinium administration, the patient developed respiratory distress with tachypnea and shallow breathing. He remained afebrile and blood pressure was maintained within normal limits on a stable dose nicardipine infusion, and he did not have associated hives or angioedema or change in urine color. At 3.5 hours after gadolinium, arterial oxygen saturation decreased to 82% and a chest radiograph demonstrated bilateral pulmonary infiltrates (Figure 1). He was administered 40 liters/minute high flow nasal cannula oxygen and 0.5 mg/kg furosemide with urine output response of 0.83 mL/kg/hr over the ensuing 6 hours. A repeat chest radiograph eight hours later was unchanged but work of breathing was increased, and the patient was administered full face mask non-invasive bilevel positive airway pressure (BiPAP) with peak inspiratory pressure 18 cmH2O and positive end expiratory pressure 8 cmH2O, FiO2 0.70. He was administered 1 mg/kg methylprednisone and cefepime was added to the antibiotic regimen. The patient’s dyspnea stabilized, and over the next four days he received a total of 3 mg/kg furosemide that produced 6 liters of urine output, becoming net negative 4 L for the hospitalization. Positive pressure ventilation was discontinued at four days after gadolinium administration. Blood cultures remained negative and the patient remained stable in room air until hospital discharge. A follow-up non-contrast brain MRI on HD50 showed marked improvement of PRES findings. The patient was discharged from the hospital on HD50 with a PICC line to complete an 8-week course of antibiotics. Please see Figure 2 for detailed hospital course timeline.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees, and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
Acute lung injury associated with administration of gadolinium-based contrast has not been previously described in a pediatric patient. Gadolinium-based contrast agents (GBCAs) are widely used in medical imaging and are typically well tolerated. Hypersensitivity reactions are rare, with mild reactions in 0.05% of patients; moderate in 0.01% and severe in 0.005% (2). Patients with hypersensitivity reactions develop signs of anaphylaxis such as hypotension, respiratory distress, and uvular edema within minutes of exposure (1). In contrast, our patient developed acute pulmonary edema and respiratory failure at three hours in the absence of hives, upper airway symptoms, diarrhea, abdominal pain, hypotension, or other signs of anaphylaxis.
To the best of our knowledge, a total of five cases of gadolinium-induced lung injury have been described in the literature, all in adult patients. The initial case reported by Demirhan et al. (2012) described an adult male who developed severe dyspnea and cyanosis immediately following gadolinium contrast administration (3). Three additional cases, reported by Park et al. (2015) (4) and Kerget et al. (2018) (1) described adult patients without atopic histories who developed angioedema and pulmonary edema 30–50 minutes following administration of intravenous gadolinium. These patients both received epinephrine and dexamethasone for anaphylaxis treatment, although the onset of their symptoms was outside the typical window of an anaphylactic reaction to gadolinium (1). Tran (2017) (5) and Lee (2019) (6) reported an additional two adult cases without atopic history who developed dyspnea and cyanosis 90 minutes following GBCA administration for abdominal MRI. Similar to our patients, neither had skin rash, angioedema, or hypotension. A 77-year-old male patient with a history of multiple strokes died from complications of pulmonary edema (5), while the previously-healthy 49 years old female patient whom Lee reported recovered following treatment with steroids and BiPAP (6). Cases are summarized in Table 2.

Full table
The mechanism of gadolinium-induced lung injury is unknown. Nephrogenic systemic fibrosis secondary to gadolinium toxicity is associated with increased NADPH oxidase 4 (Nox4) and CD34+ fibroblasts within the dermis (7). In acute respiratory distress syndrome (ARDS) mouse models, upregulation of Nox4 is correlated with increased permeability of endothelial cells and more severe lung injury (8). Gadolinium deposition in lungs associated with pulmonary fibrosis has been identified in autopsy specimens, and it is conceivable that that gadolinium induces NADPH oxidase in lung tissue, increasing capillary permeability and resulting in pulmonary edema (9). However, to oir knowledge this mechanism has not been shown in humans or an animal model of acute gadolinium-induced respiratory failure.
Our patient developed moderate ARDS according to the PALICC definition by ninety minutes after gadolinium administration (10). He did not have symptoms of anaphylaxis, such as skin rash, angioedema, or hypotension, making an Ig-E mediated hypersensitivity reaction unlikely. Aspiration pneumonitis is also less likely without suspected aspiration during MRI, in the absence of fevers and without focal infiltrates in dependent lung fields. Although the patient received a total of 10 mL/kg fluid bolus and 32mg/kg total dose of propofol for MRI, cardiogenic pulmonary edema is unlikely given an echocardiogram demonstrating normal systolic cardiac function and lack of diastolic dysfunction earlier in the day, a total fluid balance near even for the hospitalization, lack of an oxygen requirement prior to gadolinium, and well-controlled hypertension on IV nicardipine. Moreover, the pulmonary edema resolved with diuretics and without a need for inotropic therapy. Diastolic dysfunction, which is difficult to accurately measure by echocardiography, could have contributed to cardiogenic edema if it were present to a significant degree, however this was not observed by echocardiogram.
Propofol syndrome is another potential cause of acute lung injury and must be considered given the high dose of propofol (19.25 mg/kg/hour) that he received. Propofol-reflated infusion syndrome (PRIS) most commonly occurs after 48 hours of propofol infusion and is diagnosed by refractory bradycardia accompanied by hepatomegaly, lipemic plasma, metabolic acidosis, and skeletal muscle damage- none of which was present in our patient (11). Furthermore, propofol toxicity usually presents with cardiac failure and metabolic acidosis rather than isolated lung injury. Our patient did not exhibit any of these other symptoms, and his normal electrolytes without increase in BUN or Cr and lack of acidosis is not consistent with PRIS.
Of note, our patient had nephrotic-range proteinurea on the day prior to MRI, most likely due to the IV contrast he received during CT angiography two days previously. Because gadolinium is renally excreted, patients with poor glomerular filtration are at increased risk of toxicity and patients with severe chronic kidney disease (eGFR <30 mL/min/1.73 m2) or acute kidney injury are advised to avoid gadolinium-based contrast (12). It is possible that our patient’s prior kidney injury pre-disposed him to have poorer gadolinium clearance, thereby increasing his risk of gadolinium accumulation. However, our patient did not meet criteria for an acute kidney injury (BUN 5, Cr 0.65; estimated GFR =96 mL/min/1.73 m2). The half-life of gadolinium is 1.3 hours in patients with normal renal function and is prolonged up to 34 hours in patients with severely reduced renal function (GFR <10 mL/min/1.73 m2), which is not consistent with our patient’s clinical status (13). Additionally, gadolinium would be unlikely to accumulate in lung tissues; indeed, dermal accumulation (known as nephrogenic systemic fibrosis) is most commonly described. In this condition, patients with renal impairment develop thickened and contracted skin, due to proliferation of CD34+ fibroblasts and myeloid cell deposition in the extracellular dermal matrix (7).
Our patient had other risk factors that may have predisposed him to developing ARDS. During the course of his hospitalization, he had bilateral pleural effusions and multifocal pneumonia; although he had been well saturated in room air and breathing comfortably prior to gadolinium administration, he may still have been predisposed to developing pulmonary capillary leak from acute gadolinium toxicity. He had also developed seizures and PRES two days prior to ARDS, both of which may have predisposed to increased capillary permeability. In animal studies, hypertensive rats are more susceptible to inflammatory lung damage (14). Neurogenic pulmonary edema is postulated to result from sympathetic adrenergic effects on capillary endothelium causing pulmonary vasodilation (15). However, this usually occurs acutely after seizures or up to 24–48 hours post-insult, outside of the time frame of our patient’s respiratory symptoms, and PRES is not normally associated with pulmonary edema (16).
Conclusions
We describe the first pediatric patient to experience acute lung injury within three hours of administration of gadolinium-based contrast. Providers should be aware that although usually well tolerated, gadolinium-based contrast can result in potentially life-threatening but reversible pulmonary edema that can be managed with positive-pressure ventilation and diuretics.
Acknowledgments
We would like to thank the patient and his family
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jeccm-20-117
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jeccm-20-117). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees, and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kerget B, Aksakal A, Erol D, et al. Respiratory Medicine Case Reports Acute respiratory distress syndrome after the use of gadolinium contrast agent. Respir Med Case Rep 2018;25:336-8. [Crossref] [PubMed]
- Rozenfeld MN, Podberesky DJ. Gadolinium-based contrast agents in children. Pediatr Radiol 2018;48:1188-96. [Crossref] [PubMed]
- Demirhan A, Tekelioglu UY, Akkaya A, et al. Magnetic resonance imaging contrast agent related pulmonary edema : a case report. Eur Rev Med Pharmacol Sci 2012;16:110-2. [Crossref] [PubMed]
- Park J, Byun IH, Park KH, et al. Acute Respiratory Distress Syndrome after the Use of Gadolinium Contrast Media. Yonsei Med J 2015;56:1155-7. [Crossref] [PubMed]
- Tran D, Cren P, Capdeville A. Œ dème pulmonaire non cardiogénique fatal après injection de gadolinium. Ann Fr Med Urgence 2017;7:396-8. [Crossref]
- Lee Y, Chung TY, Liu H. A rare case of acute respiratory distress syndrome caused by use of gadolinium-based magnetic resonance imaging contrast media. Respirol Case Rep 2019;7:e00483 [Crossref] [PubMed]
- Leyba K, Wagner B. Gadolinium-based contrast agents : why nephrologists need to be concerned. Curr Opin Nephrol Hypertens 2019;28:154-62. [Crossref] [PubMed]
- Palumbo S, Shin Y, Ahmad K, et al. Dysregulated Nox4 ubiquitination contributes to redox imbalance and age-related severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2017;312:L297-L308. [Crossref] [PubMed]
- Sanyal S, Marckmann P, Scherer S, et al. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis - An autopsy-based review. Nephrol Dial Transplant 2011;26:3616-26. [Crossref] [PubMed]
- Cheifetz IM. Pediatric ARDS. Respir Care 2017;62:718-31. [Crossref] [PubMed]
- Chidambaran V, Costandi A, D’Mello A. Propofol: A Review of its Role in Pediatric Anesthesia and Sedation. CNS Drugs 2015;29:543-63. [Crossref] [PubMed]
- Nardone B, Saddleton E, Laumann AE, et al. Pediatric nephrogenic systemic fibrosis is rarely reported : a RADAR report. Pediatr Radiol 2014;173-80. [Crossref] [PubMed]
- Ledneva E, Karie S, Launay-vacher V, et al. Renal Safety of Gadolinium- based Contrast Media in Patients with Chronic Renal. Radiology 2009;250:618-28. [Crossref] [PubMed]
- Liu DD, Hsu YH, Chen HI. Endotoxin-induced acute lung injury is enhanced in rats with spontaneous hypertension. Clin Exp Pharmacol Physiol. 2007;34:61-9. [Crossref] [PubMed]
- Su CF, Kao SJ, Chen HI. Acute respiratory distress syndrome and lung injury: pathogenetic mechanism and therapeutic implication. World J Crit Care Med 2012;1:50-60. [Crossref] [PubMed]
- Busl KM, Bleck TP. Neurogenic Pulmonary Edema. Crit Care Med 2015;43:1710-5. [Crossref] [PubMed]
Cite this article as: Lucas A, Mohan G, Winkler A, Gardner K, Whalen M. Acute lung injury following gadolinium contrast: a case report. J Emerg Crit Care Med 2021;5:18.