How does adequacy of caloric and protein intake associate with the clinical outcomes in critically ill adults of high nutritional risk?
Introduction
Background
For years, there have been numerous debates about different aspects of nutritional support among critically ill patients—from timing of initiation, route and dose of artificial nutrition; to the optimal rate of progression toward nutritional goal and individualization of nutritional strategies in special subgroups (1-3). There are currently four international guidelines to address these aspects (4-7) yet the level of evidence varies and they mostly comprise observational studies or expert opinion. What is certain though is that critically ill patients deserve special attention on nutritional therapy as they experience a series of metabolic adaptations in response to the acute physiological stress. While these responses are thought to provide evolutionary advantage in overcoming survivable insults, if prolonged and exaggerated they become self-destructive and cause secondary metabolic damage (8,9).
In one review article, the prevalence of malnutrition among critically ill patients reaches nearly 80%. Such problem is notoriously associated with poor clinical outcomes (10). Ironically, majority of the critically ill patients fail to receive adequate nutritional intake (11). While meeting caloric target appears an instinct (12), recent attention has been on the role of protein in mitigating acute illnesses and hastening long-term recovery (2,13,14). Given the complexity of nutritional intervention in critical care, nutritional risk assessment is proposed as a routine to guide subsequent nutritional strategy (15).
Owing to the limitations in obtaining detailed weight and diet history, and accurate anthropometric data among critically ill patients, a novel nutritional assessment tool called NUTrition Risk in the Critically Ill (NUTRIC) score was developed by Heyland et al. in 2011 (16) and was subsequently validated for use in different populations including Chinese (17-19). Being fast and pragmatic, and with a good discriminating value, it is increasingly being utilized around the world (20).
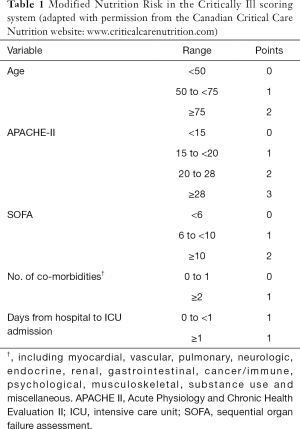
The NUTRIC score combines prehospitalization parameter (age), acute starvation status (prehospital admission duration), acute [interleukin (IL)-6] and chronic inflammatory parameters (No. of comorbidities), and severity of illness [Acute Physiology and Chronic Health Evaluation (APACHE)-II; and Sequential Organ Failure Assessment (SOFA) scores] on ICU admission to identify patients at risk of developing adverse outcomes and who may otherwise benefit from aggressive nutrition therapy. Without using IL-6 values, it is called modified NUTRIC (mNUTRIC) score. A total score of 0 to 4 correspond to low nutritional risk whereas 5 to 9 correspond to high nutritional risk. Table 1 shows the variables and point distribution of the mNUTRIC scoring system.
Full table
Various studies have shown that provision of greater caloric and protein intake to the high-risk critically ill patients confer outcome benefit in terms of mortality and morbidity (18,19,21). Conversely, these patients are more likely to experience harm if there is inadequate nutritional support (22). However, this has not been proven in our local population.
Study objective and hypothesis
This is the first study in Hong Kong which aims to examine the association between nutritional adequacy and all-cause 60-day mortality among critically ill adult patients of high nutritional risk admitted to a local ICU of a tertiary hospital. In addition, it also investigates the association of caloric and protein adequacy with other patient-oriented outcomes including renal support and mechanical ventilation. We hypothesized that in high-risk ICU patients (represented by an mNUTRIC score of 5 to 9), prescribing more of both calories and protein at two thirds or more of their respective target is associated with decreased mortality and morbidities.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jeccm-20-135).
Methods
Study design and patient selection
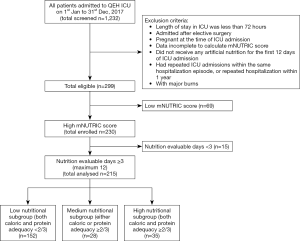
This is a retrospective single-centered cross-sectional study conducted in a 24-bed medical and surgical adult ICU of a tertiary hospital in Hong Kong (Queen Elizabeth Hospital, Kowloon). All patients admitted from January to December 2017 were screened to minimize selection bias. They were excluded if they meet any of the following: length of stay in ICU for less than 72 hours; admitted after elective surgery as they were likely to have received nutritional screening and supplemental nutritional support preoperatively; pregnant at the time of ICU admission; data were incomplete to calculate mNUTRIC score; did not receive any artificial nutrition for the first 12 days of ICU admission; had repeated ICU admissions within the same hospitalization episode; had repeated hospitalization within 1 year; or with major burns due to local referral policy. Among the remaining ones who were eligible, patients with high mNUTRIC of 5 to 9 were recruited, and those who had at least three nutrition evaluable days were selected and categorized into low, medium and high nutritional subgroup according to their caloric and protein adequacy. Figure 1 illustrates the process of patient selection.
Data collection
There was no intervention in this observational study. All nutritional therapies were at the discretion of the attending clinicians. Patient’s information including baseline and nutritional characteristics were retrieved from the Computer Information System, Hospital Authority Clinical Management System and electronic Patient Record. Clinical outcomes were analysed up to 60 days counting from the date of ICU admission. Investigators involved in the procedure of data collection were primed and the chance for information bias was deemed minimal.
Independent variables
Baseline characteristics
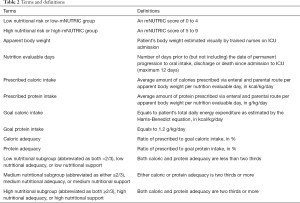
Table 2 shows the definitions for specific terms used in the current study. Apparent body weight, although less accurate than anthropometric measurement, is a practical and common way of weight estimation in ICU. When compared to derived values, visual estimation by trained personnel allows a closer approximation to patient’s actual body weight especially those of extreme body sizes. It is thought to give a more realistic estimation of the nutritional requirement. Other baseline characteristics collected were age, sex, body mass index (BMI), APACHE-II and SOFA scores, days in hospital prior to ICU admission, number of co-morbidities if any.
Full table
Nutritional characteristics
The period of nutrition evaluable days is believed to represent the acute phase of nutritional status among the critically ill as most patients would only start oral diet after stabilization and shortly before transfer to general ward. The total amount of calories and protein prescribed were calculated by multiplying the volume of artificial nutrition given both enterally and parenterally by the energy (in kcal) and protein (in g) content per 1 mL of standard formula. Caloric intake from dextrose solution and propofol (if more than 200 mg per hour over 24 hours) were also taken into account. “Prescribed” but not the “actual” amount of artificial nutrition absorbed by the patient was considered due to practical reason in this study.
The goal caloric intake was based on the Harris-Benedict predictive equation taking into account the most significant stress factor and activity factor. On the other hand, a goal protein intake of 1.2 g/kg/day is being adopted according to the latest guidelines and consensus (4,7,23,24). Taking two thirds as the lower threshold of caloric and protein adequacy (12), three nutritional subgroups were derived: low (both <2/3), medium (either ≥2/3) and high (both ≥2/3). There was limited access to indirect calorimetry in our unit, and nitrogen balance measurement is not routinely performed.
Following data were also collected: time from ICU admission to start of nutritional support; initial route of nutritional support being enteral (versus parenteral); any major intra-abdominal or gastrointestinal (GI) system related factors unfavourable for enteral feeding (such as paralytic ileus, intra-abdominal hypertension, mechanical intestinal obstruction, severe diarrhoea, major and/or active gastrointestinal bleeding, ischemic bowel, and recent bowel operation); and presence of GI intolerance (defined as gastric residual volume of 500 mL or more over 4 hours, or so documented in physician notes).
Dependent variables
The primary outcome was all cause 60-day in-hospital mortality since ICU admission. If a patient was discharged home or transferred to a private hospital before day 60, that patient would be considered alive on day 60. The secondary outcomes were: need of renal support (in any modalities of dialysis regardless of pre-existing end stage renal failure); presence of bloodstream infection from culture (excluding skin contaminants); need of hemodynamic support (either pharmacologically or mechanically, of any dose and any duration); need of mechanical ventilation (regardless of the cause and timing); and total duration of mechanical ventilation for 7 days or more.
Sample size
Assuming a medium effect size (Cohen’s f =0.25) for the maximum reduction in 60-day mortality among high-mNUTRIC patients of different nutritional adequacies (18,19,21), a total sample size of at least 159 would allow a two-tailed significance level of 5% and statistical power of 80% using the G*power calculator (version 3.1.9.4; Heinrich Heine University Düsseldorf, Düsseldorf, Germany). Considering possible dropout due to incomplete data (10%), minimal duration of nutrition evaluable days (5%), and loss to follow-up as a result of short ICU stay (10%), 212 patients were to be enrolled in this study.
Statistical analysis
Results were presented as mean ± standard deviation (SD) for continuous variables and frequency (percent) for categorical variables. For the overall comparison of the baseline and nutritional characteristics, and clinical outcomes among the three nutritional groups, a one-way analysis of variance (ANOVA) was used. Bonferroni’s post hoc analysis with correction was used for multiple between-group comparisons of the continuous variables that showed a significant difference in the overall comparison. For the comparison of categorical variables, Pearson’s Chi-square test or Fisher’s exact test was used. The overall and risk-free survivals up to 60 days were calculated using the Kaplan-Meier method, and differences were compared using the log-rank test. Cox regression models were performed using multivariate analysis in order to illustrate the relationship between nutritional adequacy and all-cause 60-day mortality. Adjustment was done according to individual risk factors and potential confounders.
Cramer’s V with chi-square was used to find out any correlation between different levels of nutritional adequacy and secondary outcomes, and to ascertain the strength of the differences in the variables.
A P value of <0.05 was considered statistically significant. All analyses were done using a statistical software package STATA (version 14.2; StataCorp., College Station, TX, USA).
Ethical principle
This study was conducted in accordance to the Declaration of Helsinki (as revised in 2013) and was approved by the Research Ethics Committee (Kowloon Central/Kowloon East) of Hospital Authority (Ref.: KC/KE-20-0194/ER-4). Individual consent for this retrospective analysis was waived.
Results
Patient characteristics
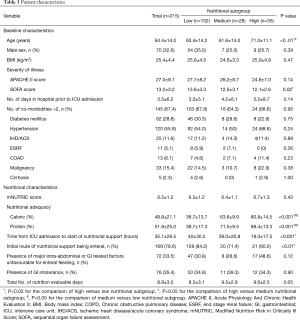
Table 3 shows the overall and subgroup patient characteristics. A total of 1,232 patients were screened and 215 patients were analysed. Most (70.7%) of the patients belonged to the low nutritional subgroup. Patients with high nutritional adequacy were significantly older (63.4±14.2 vs. 61.6±14.0 vs. 71.0±11.1 years, P<0.01) while those with low nutritional adequacy were significantly more ill as suggested by the SOFA score (13.6 ± 3.3 vs. 12.5±3.1 vs. 12.1±2.6, P=0.02) although the absolute difference is small and the APACHE-II scores did not differ significantly among the three subgroups. Otherwise, the three nutritional subgroups were similar in their baseline characteristics.
Full table
As for the nutritional characteristics, the high nutritional subgroup was significantly more likely to receive early nutritional support when compared to low nutritional subgroup (time from ICU admission to start of nutritional support =40±30.2 vs. 28.0±20.8 vs. 19.3±17.5 hours, P<0.001). The difference in mean time for initiation of artificial nutrition between the two subgroups was about one day. On the other hand, the low nutritional subgroup was significantly more likely to start nutrition via the enteral route when compared to high nutritional subgroup (84.2% vs. 71.4% vs. 60%, P<0.01).
Clinical outcomes
Primary and other relevant outcomes
Table 4 shows the mortality and length of stay outcomes, and Figure 2 illustrates the Kaplan-Meier curves up to 60 days after ICU admission. There was no significant difference in all-cause 60-day mortality among the three nutritional subgroups, with an overall rate of 37.7% (81/215). Other relevant clinical outcomes including ICU mortality (21.9% for all), ICU length of stay (13.4±11.4 days for all) and hospital length of stay (38.5±34.8 days for all) also did not differ among the three nutritional subgroups.
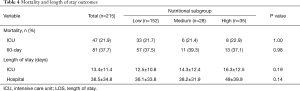
Full table
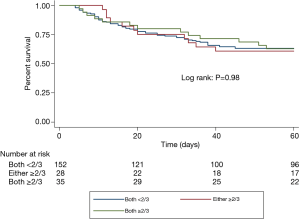
Table 5 shows the results of Cox regression models for all-cause 60-day mortality. Taking the low nutritional subgroup as reference, the hazard ratios of both medium and high nutritional subgroups did not reach statistical significance, whether adjusted or unadjusted.
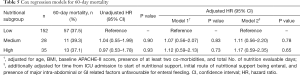
Full table
Secondary outcomes
Table 6 shows the secondary outcomes and their correlation with different levels of nutritional adequacy. Almost all patients required mechanical ventilatory support (98.1%) and a substantial proportion of them required hemodynamic support (87%). Those of high nutritional adequacy were significantly more likely to have prolonged mechanical ventilation (58.6% vs. 78.6% vs. 85.7%, P<0.005) and the strength of association was moderate (Cramer’s V =0.23). There appeared a trend towards a greater need of renal support in the low nutritional subgroup (53.9% vs. 35.7% vs. 37.1%, P=0.07) although the strength of association was weak (Cramer’s V =0.16).
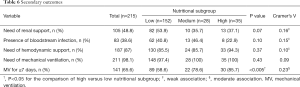
Full table
Table 7 shows the results of a post hoc multivariate analysis for prolonged mechanical ventilation. Among the three nutritional subgroups, the adjusted OR for high nutritional adequacy was up to 7.26 and was statistically significant (P=0.03, 95% CI 1.24–42.57). However, the 95% CI was wide suggesting the possibility of a false positive result. A dose-response analysis was technically difficult considering the categorical nature of the variables and the small sample size of the study.

Full table
Discussion
In our current study, the all-cause 60-day mortality did not differ significantly among the three nutritional subgroups, and it had no significant association with different levels of nutritional adequacy. The chance of prolonged mechanical ventilation was significantly higher among those of high nutritional support with a moderate strength of association. This is contrary to the common belief that more nutrition is universally better for patients at risk of malnutrition although it echoes with the finding from some of the latest study.
Indeed, current opinion on the dose and timing of nutritional therapy in the critically ill is dividing (1,3,25). Although a number of studies suggest a dose-related beneficial relationship between the amount of calories and/or protein and mortality outcome especially in patients of high nutritional risk, all are observational in nature and some are single-centered with small sample sizes (12,13,18,19,21,22). Lee et al. prospectively observed within a mixed Asian cohort that when stratified according to nutritional risk, the mortality of patients with high nutritional risk did not significantly differ among those who received both ≥2/3 and either ≥2/3 compared with both <2/3 of energy and protein prescription (26). This was similar to the finding of our study. However, caution should be exercised when interpreting results from a single-centered observational study which in itself has limited generalizability.
Of note, randomized controlled data have demonstrated that near-target caloric intake in a mixed cohort of ICU patients actually do more harm (27), and a J-shaped relationship between caloric and survival in critically ill patients was being proposed (28). Such divergent results may be partly explained by the heterogeneity in the methodological characteristics of nutrition-related research studies. This is further hindered by the fact that even within the same critically ill population, their metabolic response and therefore clinical consequences to exogenous nutrients may be different, not to mention that the nutritional status of an individual patient will likely change as the clinical course unfolds.
This leads to the question of how one metabolically responds to critical illness. A classical description would be the “ebb and flow” phases first proposed by Sir Cuthbertson in year 1942 (29). It is characterized by firstly a hypometabolic and later a hypermetabolic period as a metabolic adaptation to acute physiological insult or stress. As catabolism becomes uncontrolled and resistance to anabolic signals develops, there appears an alteration in the energy expenditure and loss of control of energy substrate use by their availability (8). Besides aggressive treatment of underlying pathologies, energy expenditure-guided nutrition therapy may be helpful in correcting the unwanted metabolic reactions and meeting the energy requirement in midst of the unavoidable mobilization of endogenous substrates (9,30).
The latest European clinical practice guideline described the different stages of critical illness as acute early (ICU day 1 to 2), acute late (ICU day 3 to 7), and recovery phase (ICU day 7 and beyond) (7). In one of the latest enteral nutrition trials randomizing 3,957 patients, augmented energy delivery (about 30 kcal/kg ideal body weight/day) in the early phase of illness was not shown to improve mortality or any secondary clinical outcomes (31) albeit criticism on the possibility of refeeding syndrome in the intervention group. Worse outcome was also found in the early parenteral nutrition group in The Early Parenteral Nutrition Completing Enteral Nutrition in Adult Critically ill Patients (EPaNIC) trial, the largest nutrition trial in critical illness (32). Notably, initial underfeeding during critical illness is not encouraged by the current literature (33-35). By recruiting patients with at least three nutrition evaluable days and limiting the duration to twelve days after ICU admission, our study effectively covered the initial period of ICU stay where the impact of nutrition therapy caused the greatest concern.
In our cohort of critically ill patients with high baseline nutritional risk, the high nutritional subgroup received almost 90% of goal calories and protein in the early course of ICU stay while the low nutritional subgroup received slightly more than a third. Whether this constitutes a higher risk of refeeding syndrome and therefore a higher prevalence of prolonged mechanical ventilation among those with high nutritional support remains arguable. Since ours is an observational study which made no attempt to influence the practice of nutrition prescription, there were only a limited number of patients of whom the caloric and protein adequacy differed in opposite directions (either was two thirds or more). It would be interesting to study the significance of energy to protein ratio in feeding the critically ill patients as more evidence is pointing towards the importance of protein rather than caloric intake during the acute phase of critical illness (13,36,37).
Since this was a retrospective single-centered observational study, no causal relationship could be established and the generalizability was limited. However, considering that this was the first local study examining the associations between nutritional adequacy and various clinical outcomes particularly focusing on critically ill adult patients of high nutritional risk, it was hypothesis-generating. Considering the non-randomisation nature of this study, potential confounders were included in the regression models to reduce bias. The goal caloric intake for individual patient was estimated by a predictive equation in this study with its inherent inaccuracy (3). At the time when data collection was done for this study, the use of indirect calorimeter was just started in our unit and was only done in selected patients. However, it is worth mentioning that although current guidelines recommend the use of indirect calorimetry to guide nutritional target (4,7), no existing data have shown its use to be more superior when compared to predictive equations in improving clinical outcomes. In conducting future local research, it would be meaningful to study the impact of nutritional therapy at different stages of critical illness (including post-ICU discharge period) with repeated assessment of patient’s nutritional requirement using standardized method and to look for the effects (as well as side effects) of nutritional interventions.
Conclusions
This was the first local study in Hong Kong examining the associations between nutritional adequacy and clinical outcomes among critically ill adult patients of high nutritional risk. These patients were often given a low level of nutritional support during the acute phase of ICU stay. The all-cause 60-day mortality did not differ significantly among the three nutritional subgroups, and it had no significant association with different levels of nutritional adequacy. The chance of prolonged mechanical ventilation was significantly higher among those of high nutritional support with a moderate strength of association although the possibility of a false positive result could not be entirely ruled out. Large-scale prospective randomized-controlled studies with energy-expenditure guided nutritional provision are solicited to substantiate the optimal nutritional therapy for this special group of ICU patients.
Acknowledgments
We would like to thank all the staff of Intensive Care Unit, Queen Elizabeth Hospital (Kowloon, Hong Kong) for their patient care and general support to this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jeccm-20-135
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jeccm-20-135
Peer Review File: Available at http://dx.doi.org/10.21037/jeccm-20-135
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jeccm-20-135). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance to the Declaration of Helsinki (as revised in 2013) and was approved by the Research Ethics Committee (Kowloon Central/Kowloon East) of Hospital Authority (Ref.: KC/KE-20-0194/ER-4). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Preiser JC, van Zanten AR, Berger MM, et al. Metabolic and nutritional support of critically ill patients: consensus and controversies. Crit Care 2015;19:35. [Crossref] [PubMed]
- Koekkoek KW, van Zanten AR. Nutrition in the critically ill patient. Curr Opin Anaesthesiol 2017;30:178-85. [Crossref] [PubMed]
- Lambell KJ, Tatucu-Babet OA, Chapple LA, et al. Nutrition therapy in critical illness: a review of the literature for clinicians. Crit Care 2020;24:35. [Crossref] [PubMed]
- McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016;40:159-211. [Crossref] [PubMed]
- Critical Care Nutrition. The Canadian clinical practice guidelines. 2015. http://www.criticalcarenutrition.com.
- Reintam Blaser A, Starkopf J, Alhazzani W, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med 2017;43:380-98. [Crossref] [PubMed]
- Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019;38:48-79. [Crossref] [PubMed]
- Preiser JC, Ichai C, Orban JC, et al. Metabolic response to the stress of critical illness. Br J Anaesth 2014;113:945-54. [Crossref] [PubMed]
- Hartl WH, Jauch KW. Metabolic self-destruction in critically ill patients: origins, mechanisms and therapeutic principles. Nutrition 2014;30:261-7. [Crossref] [PubMed]
- Lew CCH, Yandell R, Fraser RJL, et al. Association Between Malnutrition and Clinical Outcomes in the Intensive Care Unit: A Systematic Review JPEN J Parenter Enteral Nutr 2017;41:744-58. [Formula: see text]. [Crossref] [PubMed]
- Heyland DK, Dhaliwal R, Wang M, et al. The prevalence of iatrogenic underfeeding in the nutritionally 'at-risk' critically ill patient: Results of an international, multicenter, prospective study. Clin Nutr 2015;34:659-66. [Crossref] [PubMed]
- Heyland DK, Cahill N, Day AG. Optimal amount of calories for critically ill patients: depends on how you slice the cake! Crit Care Med 2011;39:2619-26. [Crossref] [PubMed]
- Weijs PJ, Stapel SN, de Groot SD, et al. Optimal protein and energy nutrition decreases mortality in mechanically ventilated, critically ill patients: a prospective observational cohort study. JPEN J Parenter Enteral Nutr 2012;36:60-8. [Crossref] [PubMed]
- Oshima T, Heidegger CP, Pichard C. Protein in nutritional support: the newborn hero for the critically ill? Crit Care 2014;18:592. [Crossref] [PubMed]
- Coltman A, Peterson S, Roehl K, et al. Use of 3 tools to assess nutrition risk in the intensive care unit. JPEN J Parenter Enteral Nutr 2015;39:28-33. [Crossref] [PubMed]
- Heyland DK, Dhaliwal R, Jiang X, et al. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care 2011;15:R268. [Crossref] [PubMed]
- de Vries MC, Koekkoek WK, Opdam MH, et al. Nutritional assessment of critically ill patients: validation of the modified NUTRIC score. Eur J Clin Nutr 2018;72:428-35. [Crossref] [PubMed]
- Mukhopadhyay A, Henry J, Ong V, et al. Association of modified NUTRIC score with 28-day mortality in critically ill patients. Clin Nutr 2017;36:1143-8. [Crossref] [PubMed]
- Rahman A, Hasan RM, Agarwala R, et al. Identifying critically-ill patients who will benefit most from nutritional therapy: Further validation of the "modified NUTRIC" nutritional risk assessment tool. Clin Nutr 2016;35:158-62. [Crossref] [PubMed]
- Reis AMD, Fructhenicht AVG, Moreira LF. NUTRIC score use around the world: a systematic review. Rev Bras Ter Intensiva 2019;31:379-85. [Crossref] [PubMed]
- Compher C, Chittams J, Sammarco T, et al. Greater Protein and Energy Intake May Be Associated With Improved Mortality in Higher Risk Critically Ill Patients: A Multicenter, Multinational Observational Study. Crit Care Med 2017;45:156-63. [Crossref] [PubMed]
- Jung YT, Park JY, Jeon J, et al. Association of Inadequate Caloric Supplementation with 30-Day Mortality in Critically Ill Postoperative Patients with High Modified NUTRIC Score. Nutrients 2018;10:1589. [Crossref] [PubMed]
- Weijs PJ. Fundamental determinants of protein requirements in the ICU. Curr Opin Clin Nutr Metab Care 2014;17:183-9. [Crossref] [PubMed]
- Leyderman I, Yaroshetskiy A, Klek S. Protein Requirements in Critical Illness: Do We Really Know Why to Give So Much? JPEN J Parenter Enteral Nutr 2020;44:589-98. [Crossref] [PubMed]
- Wernerman J, Christopher KB, Annane D, et al. Metabolic support in the critically ill: a consensus of 19. Crit Care 2019;23:318. [Crossref] [PubMed]
- Lee ZY, Noor Airini I, Barakatun-Nisak MY. Relationship of energy and protein adequacy with 60-day mortality in mechanically ventilated critically ill patients: A prospective observational study. Clin Nutr 2018;37:1264-70. [Crossref] [PubMed]
- Arabi YM, Haddad SH, Tamim HM, et al. Near-target caloric intake in critically ill medical-surgical patients is associated with adverse outcomes. JPEN J Parenter Enteral Nutr 2010;34:280-8. [Crossref] [PubMed]
- Crosara IC, Melot C, Preiser JC. A J-shaped relationship between caloric intake and survival in critically ill patients. Ann Intensive Care 2015;5:37. [Crossref] [PubMed]
- Cuthbertson DP, Angeles Valero Zanuy MA, Leon Sanz ML. Post-shock metabolic response. 1942. Nutr Hosp 2001;16:176-82; discussion 175-6. [PubMed]
- Lheureux O, Preiser JC. Role of Nutrition Support in Inflammatory Conditions Nutr Clin Pract 2017;32:310-7. [Formula: see text]. [Crossref]
- Chapman M, Peake SL, Bellomo R, et al. Energy-Dense versus Routine Enteral Nutrition in the Critically Ill. N Engl J Med 2018;379:1823-34. [Crossref] [PubMed]
- Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med 2011;365:506-17. [Crossref] [PubMed]
- Singer P, Pichard C, Heidegger CP, et al. Considering energy deficit in the intensive care unit. Curr Opin Clin Nutr Metab Care 2010;13:170-6. [Crossref] [PubMed]
- Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs. full enteral feeding in patients with acute lung injury: the EDEN randomized trial. Jama 2012;307:795-803. [Crossref] [PubMed]
- Aldridge K. Permissive underfeeding or standard enteral feeding in critically ill adults. J Intensive Care Soc 2015;16:348-9. [Crossref] [PubMed]
- Hoffer LJ, Bistrian BR. Appropriate protein provision in critical illness: a systematic and narrative review. Am J Clin Nutr 2012;96:591-600. [Crossref] [PubMed]
- Nicolo M, Heyland DK, Chittams J, et al. Clinical Outcomes Related to Protein Delivery in a Critically Ill Population: A Multicenter, Multinational Observation Study. JPEN J Parenter Enteral Nutr 2016;40:45-51. [Crossref] [PubMed]
Cite this article as: Chan KL, Au SY, Ng WY. How does adequacy of caloric and protein intake associate with the clinical outcomes in critically ill adults of high nutritional risk? J Emerg Crit Care Med 2021;5:12.