
Progression of aortic intramural hematoma with associated penetrating aortic ulcers with medical management requiring surgical management case report
Introduction
Acute aortic syndrome (AAS) is an umbrella term for aortic pathologies encompassing the classic aortic dissection, intramural hematoma (IMH), contained aortic rupture, and penetrating aortic ulcer (PAU) (1). A PAU refers to an ulceration which penetrates the media of the aortic wall. The Debakey and The Stanford classification are two widely recognized classification systems for AAS (2,3). Out of the two, the Stanford classification is more commonly used in most clinical settings. The Stanford classification is based on the location of the lesion and classifies lesions in the ascending aorta as type A and the rest as type B (3). In this case, the patient presents with a unique mix of different AAS which progressed despite medical management, eventually requiring surgical intervention. The distinction between an IMH and aortic dissection in treatment is not widely recognized, nor is the treatment of PAUs. We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jeccm-20-153).
Case presentation
A 50-year-old African American male with a history of uncontrolled hypertension, not currently taking any medications for the past month, presented to the emergency room with shortness of air for the past 2 weeks with headache and blurry vision. He was found to be severely hypertensive with pressure readings no less than 250/150 mmHg, a heart rate of 77 beats per minute, and an oxygen saturation of 97% on room air. Patient had tested negative for COVID-19 one week prior. Upon evaluation by the ICU team, patient denied headache, dizziness, vision changes, chest pain, palpitations, syncope, abdominal pain, nausea, vomiting, cough, or sweats. Past medical history was significant only for hypertension and a family history of hypertension, CVA, and end-stage renal disease in his father. He had no prior relevant interventions. Social history was significant for use of cigars once a month with no other illicit substance use. Patient was active at baseline with either running or walking miles with his dogs daily. He worked as a barber with no heavy lifting or increased stress at the work place. Patient took no medications. His entire physical exam including cardiovascular and respiratory auscultation was unremarkable. Patient was transferred to ICU care. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Consent to writing and publishing this case report was obtained from the patient both verbally and through written consent.
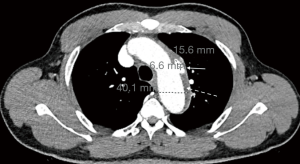
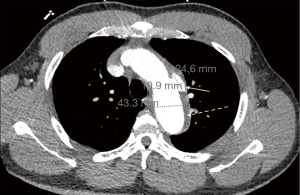
Initial workup showed an elevated creatinine of 3.74 mg/dLwith an unknown baseline, negative troponin-T x1, and a negative UDS. A Chest X-ray showed prominent atherosclerotic thoracic aorta that prompted a computed tomography angiography (CTA) of chest was per the “Dissection Protocol”. CTA revealed a penetrating lateral aortic arch ulcer measuring 2 cm by 9 mm in transverse dimension and 1.5 cm craniocaudad dimension. A second, smaller penetrating ulcer closely adjacent, along the inferior margin of the aortic arch measuring 6.6 mm ×15.6 mm ×8 mm was also seen. CTA also showed an IMH 9 mm in thickness along the aortic arch beginning just past the origin of the left subclavian artery and extending to the distal aspect of the aortic arch (Figure 1). The CTA abdomen/pelvis per “Dissection Protocol” showed no flow-limiting stenosis, dissection, or aneurysm of the aorta. EKG shows prolonged QTc interval of 505 ms with left ventricular hypertrophy. The patient was immediately treated for hypertensive emergency and started on an esmolol and nicardipine drip with a target decrease of mean arterial pressure by 20% in the first hour, followed by 10% over the next 23 hours. Vascular surgery was consulted and recommended medical management with a repeat CTA of chest after 48 hours. Fortunately, the patient did not face any diagnostic challenges due to socioeconomic determinants of health. The patient’s hypertension was very resistant and necessitated titrating up to maximum doses of multiple oral medications in order to wean patient off the intra-venous anti-hypertensives. The patient’s blood pressure was controlled on oral carvedilol 25 mg twice a day, nifedipine 120 mg once a day, clonidine 0.3 three times a day with the addition of IV labetalol 10 mg and IV hydralazine 10 mg to used as needed. The patient continued to remain completely asymptomatic. The repeat CTA of chest 48 hours showed an increase in size of the largest multi-lobulated irregular penetrating ulcer to 24.6 mm ×10.9 mm ×1.7 mm and the smaller adjacent ulcer to 10.3 mm ×21 mm ×8 mm. There was also re-demonstration of liquefying IMH along the aortic arch (Figure 2) and proximal descending thoracic aorta with associated aneurysmal dilation of the same areas. Extension of the hematoma to the origin of the left common carotid artery was difficult to exclude. Vascular surgery performed thoracic endovascular aortic repair (TEVAR) on hospital day 4. Gore C-TAG active control device was used for the TEVAR. Patient continued to be asymptomatic after surgery with controlled blood pressures. Work-up for secondary hypertension was underway prior to transfer out of the ICU. Patient plans to follow up with vascular surgery outpatient, along with a new primary care provider and nephrology.
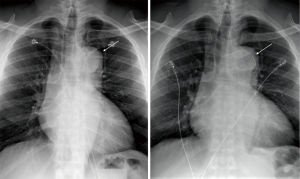
As for follow-up, a chest radiograph was done for fever a week post-op which showed postsurgical changes of endovascular stent graft involving the aortic arch and proximal descending thoracic aorta with stable appearance of the thoracic aorta (Figure 3). At a 20-day post-operative visit, vascular surgery requested a CTA chest 6 months after TEVAR procedure. Of recently, patient’s blood pressures are at goal with carvedilol, amlodipine, clonidine, furosemide, and lifestyle modifications. This is assessed with follow-up visits and blood pressure logs. Patient has been diagnosed with CKD Stage IV and follows with nephrology. Routine lab workup for secondary hypertension was negative. Patient follows with Sleep Clinic to evaluate for obstructive sleep apnea. There have been no adverse or unanticipated events thus far. Over a recent phone call, patient reports that he is very appreciative of the care he received in the ICU and that he feels much improved. He is motivated to stay on top of his outpatient appointments.
Discussion
Differentiating between the aortic syndromes, especially IMH and aortic dissection has been controversial which can lead to confusion in approaching treatment in the real-world setting.
Aortic dissection is a tear in the aortic intima which allows blood between the layers of the vessel wall, thus creating an intimal flap and divides the aorta into a true and false lumen (4). It is common clinical practice that any of the type B aortic dissection are initially treated with medical management while endovascular intervention is reserved for patients who have complications or progression of the type B dissection. Type A acute dissection are repaired emergently using open surgical techniques.
Acute IMH was first described by Krukenberg in 1920 (5)with the main difference being there is an absence of tunica intima disruption and hematomas are likely due to vaso-vasorum injury. Per literature review, the appropriate management of an IMH is not as well defined nor as understood as that of classic dissection primarily because hematomas can stabilize, regress, resolve, or progress.
PAUs are typically associated with extensive vascular atherosclerosis disease and hypertension, caused by ulceration of an aortic atherosclerotic lesion which penetrates the internal elastic media into the media (6). PAUs may be associated with a hematoma within the media and may progress to perforation or aortic dissection.
A meta-analysis by Maraj et al. found that 143 IMHs showed that 57% were type A and 43% were type B, and 94% had a non-traumatic cause (7). According to the IRAD review, IMH is more common in the descending aorta compared to aortic dissection, 60% vs. 35% respectively (8).The distinction between an IMH and aortic dissection is paramount as both differ in treatment and clinical complications. Type B IMH treated similarly to a type B dissection is generally acceptable except when the IMH is associated with a factor of progression or is a complicated course, for which earlier intervention may be needed to prevent complications. Type B IMH progress to aortic rupture, hematoma expansion, or dissection in 8–16% of patients. One of the predictors of progression are the presence of PAUs (8,9). Other predictors of progression, despite adequate medical management, include maximum aortic diameter ≥40 mm, maximum aortic thickness ≥10 mm on CT scan, >70 years of age, and presence of a PAU (10). Sebastià et al. believe signs of a complicated course can be presence of continuous chest pain despite medical treatment, hemodynamic instability, signs of aortic rupture, presence of large ulcer like projections >10 mm, maximum aortic diameter >55 mm, or rapid aortic growth while in the hospital (11). These patients would undergo endovascular treatment or surgical treatment if the former was contraindicated. Timperley and Banning show that an IMH associated with a PAU has a higher rate of progression with medical therapy. This is illustrated in their series of 65 patients with IMH where 31 of them had type B IMH with associated PAU. Progression with medical therapy occurred significantly more often in those with associated PAU than those who did not (48% vs. 8%) (12). In these patients, early endovascular intervention should be used to treat these lesions and prevent complications. In a small series of 26 patients, all had successful endovascular repair with 3 patients who died within 30 days and 2 had an early leakage of blood around the graft. Overall, the actuarial survival estimates at 1, 3, and 5 years were 85%, 76%, and 70%, respectively (13).
In this case report, the patient had a type B aortic IMH with PAUs located in the aortic arch, which is a unique presentation since 85–95% of PAUs are located in the descending thoracic aorta (13). This PAU classification has an increased risk of rupture and generally do not follow benign courses, requiring earlier intervention. The patient in this case had a type B IMH from non-traumatic cause likely to be from his uncontrolled resistant hypertension. Furthermore, this patient with type B IMH was initially treated for hypertensive emergency with gradual pressure reduction likely due to the unclear nature of how to treat IMH once dissection had been ruled out. Then the patient was transitioned to medical management of a type B dissection. This is generally acceptable unless patient has increased risk of IMH progression, which this patient had. Due to the size of his PAU and his young age placing him at high risk for developing complications later on, patient underwent endovascular intervention around hospital day 4 at which point there was already progression of his acute aortic pathologies.
Limitations of this case report include the varying opinions per author when it comes to the defining AAS and also the treatment. PAUs are strikingly similar to ulcer-like projections (ULP), clinical overlap between the two has provoked confusion regarding prognosis and management. Therefore, true prevalence of PAU in literature is not fully known since they could have been ULPs in previous studies, misguiding possible management (7). Another limitation is the constant new flood of guidelines regarding TEVAR intervention in acute aortic disease. The newest guidelines from October 2020 state briefly that the treatment of PAU is guided by size and symptom, lending towards the option of surgical intervention being more case by case (14).
The patient’s mixed AAS picture allows this case to be a prime example of what may occur when there is a lack of distinction between treatment of the different AAS. This case portrays the progression of the patient’s IMH and PAU when the components are not treated separate of aortic dissection. Firstly, it is important that the literature and knowledge regarding management of IMH be easily accessible and clear. Secondly, the association of IMH and penetrating ulcer implies a more malignant course, necessitating early endovascular or surgical intervention. It is important to not treat a type B IMH as a type B dissection without accounting for the total clinical picture and factors of progression. This case report corroborates the belief that early endovascular intervention is needed for type B IMH with associated PAUs due to the increased risk of progression. With medical management, this patient had progression of his IMH where it could not be excluded that it had extended to the origin of the left common carotid artery from the left subclavian artery. He also had widening of both transverse arch PAUs, all within 48 hours along with no changes in his asymptomatic clinical status. This patient showed definite increase in size of his PAUs while trying to optimize blood pressure with medical management, which may increase the risk of rupture.
This case report also addresses the challenge clinicians face with unclear delineation of treatment between different AAS. It is natural for physicians to treat all the AAS similar to aortic dissection since there are not well-known clinical guidelines to refer to. Also, for medicine and emergency physicians, it is important to realize that early involvement of vascular surgeons is of paramount importance. This case study also emphasizes the fact that association of an IMH with a PAU has increased risk to progress to dissection, hematoma expansion, or rupture. We present the less well known AAS such as aortic IMH and PAUs. This case demonstrates how a type B IMH, when associated with penetrating ulcers, may follow a more malignant course, and should be considered for early surgical intervention. This case illustrates the importance of understanding the distinction between all of the AAS and how treatment differs based on Stanford classification and risk factors of progression.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jeccm-20-153
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jeccm-20-153). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Consent to writing and publishing this case report was obtained from the patient both verbally and through written consent
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vilacosta I, Aragoncillo P, Cañadas V, et al. Acute aortic syndrome: A new look at an old conundrum. Postgrad Med J 2010;86:52-61. [Crossref] [PubMed]
- Debakey ME, Henly WS, Cooley DA, et al. Surgical Management of Dissecting Aneurysms of the Aorta. J Thorac Cardiovasc Surg 1965;49:130-49. [Crossref] [PubMed]
- Daily PO, Trueblood HW, Stinson EB, et al. Management of acute aortic dissections. Ann Thorac Surg 1970;10:237-47. [Crossref] [PubMed]
- Teo EP, Isselbacher EM. Essential Echocardiography. In: Valvular Heart Disease: A Companion to Braunwald’s Heart Disease. Amsterdam: Elsevier, 2019;354-68.
- Krukenberg E. Beitrage zur frage des aneurysma dissecans. Beitr Pathol Anat Allg Pathol 1920;67:329-51.
- Coady MA, Rizzo JA, Elefteriades JA. Pathological variants of thoracic aortic dissections. Penetrating atherosclerotic ulcers and intramural hematomas. Cardiol Clin 1999;17:637-57. [Crossref] [PubMed]
- Maraj R, Rerkpattanapipat P, Jacobs LE, et al. Meta-analysis of 143 reported cases of aortic intramural hematoma. Am J Card 2000;86:664-8. [Crossref] [PubMed]
- Evangelista A, Mukherjee D, Mehta RHInternational Registry of Aortic Dissection (IRAD) Investigators, et al. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation 2005;111:1063-70. [Crossref] [PubMed]
- Evangelista A, Czerny M, Nienaber C, et al. Interdisciplinary expert consensus on management of type B intramural haematoma and penetrating aortic ulcer. Eur J Cardiothorac Surg 2015;47:209-17. [Crossref] [PubMed]
- Cambria R. Analysis of predictive factors for progression of type B aortic intramural hematoma with computed tomography. J Vasc Surg 2002;35:1295-6. [Crossref] [PubMed]
- Sebastià C, Pallisa E, Quiroga S, et al. Aortic Dissection: Diagnosis and Follow-up with Helical CT. Radiographics 1999;19:45-60. [Crossref] [PubMed]
- Timperley J, Banning AP. Prognosis of Aortic Intramural Hematoma with and Without Penetrating Atherosclerotic Ulcer: A Clinical and Radiological Analysis. Circulation 2003;107:e63 [PubMed]
- Demers P, Miller DC, Mitchell RS, et al. Stent-graft repair of penetrating atherosclerotic ulcers in the descending thoracic aorta: Mid-term results. Ann Thorac Surg 2004;77:81-6. [Crossref] [PubMed]
- Czerny M, Pacini D, Aboyans V, et al. Current options and recommendations for the use of thoracic endovascular aortic repair in acute and chronic thoracic aortic disease: an expert consensus document of the European Society for Cardiology (ESC) Working Group of Cardiovascular Surgery, the ESC Working Group on Aorta and Peripheral Vascular Diseases, the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2021;59:74-9. [Crossref] [PubMed]
Cite this article as: Shah K, Ahmad H, Wilson JE, Goyal M, Dubin S. Progression of aortic intramural hematoma with associated penetrating aortic ulcers with medical management requiring surgical management case report. J Emerg Crit Care Med 2021;5:25.


