
Subcutaneous and mediastinal emphysema, uncommon complications of COVID-19 ARDS: a case series
Introduction
We report four patients, average age of 52.8±2.2 years (Table 1), all diagnosed with coronavirus disease 2019 (COVID-19) pneumonia, noted to have markedly elevated D-dimer levels (>20 mcg/mL) with negative procalcitonin levels (<1.0 ng/mL). All patients adhered to lung protective strategy (LPS) mechanical ventilation (MV), targeting plateau pressure (Pplat) of ≤30 cmH2O and driving pressure (∆P) of ≤15 cmH2O. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jeccm-20-149).
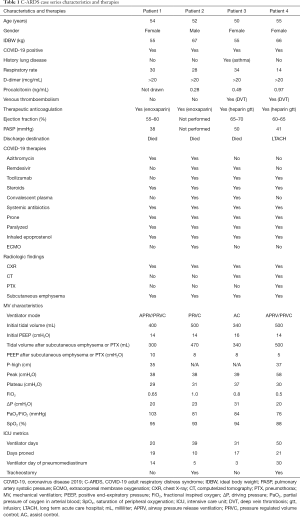
Full table
Case presentation
Despite these protective strategies, extensive pneumomediastinum in all four patients was noted on either chest X-ray (CXR) or computerized tomography (CT) of the chest, with one patient noted to have small apical pneumothorax (PTX) bilaterally (Figures 1,2). Retrospective review of all charts revealed pneumomediastinum and or PTX occurred as early as MV day 3 and as late as day 30. All except patient 3 did not have a preexisting lung condition, of which patient 3 had a history of asthma. Three of the four patients had mild to moderate pulmonary hypertension, with preserved left and right systolic function. Due to rapidly worsening COVID-19 adult respiratory distress syndrome (C-ARDS), difficulty maintaining LPS was documented in the chart, with ∆P’s ≥20 cmH2O and Pplat’s ≥30 in three out of four patients. Strategies to continue to adhere to LPS standards and ∆P’s <15 were attempted, in addition to decreasing positive end-expiratory pressure (PEEP) in all four patients (Table 1). All patients were proned, paralyzed, trialed on inhaled epoprostenol, received systemic steroids, systemic antibiotics, therapeutic anticoagulation and one or more of the following COVID-19 antiviral or immune modulator therapies: azithromycin, remdesivir, tocilizumab or convalescent plasma. Patients received variable ventilator modes between assist control (AC), pressure regulated volume control (PRVC) and airway pressure release ventilation (APRV). Average ventilator days for four patients was 13±12 days, with two of the four patients undergoing tracheostomy.
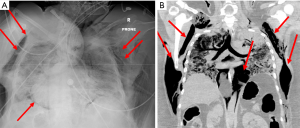
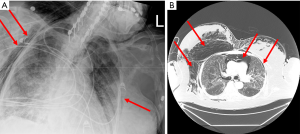
Three of the four patients died within the hospitalization, with one patient transferring to long-term acute care facility. The Institutional Review Board (IRB) approved this study and waived the need for consent from individual patients owing to the retrospective nature of the study (IRB ID: STUDY0003052). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013).
Discussion
Patients undergoing MV are at risk for ventilator-induced lung injury (VILI), such as barotrauma, volutrauma, atelectrauma, ergotrauma and biotrauma (1,2). The latter is less understood, in relationship to COVID-19 infection, as profound cytokines release syndrome may further elevate interleukin-6 (IL-6) levels (1,3). Regardless of the exact mechanism of VILI, multiple clinical trials have demonstrated that injured lungs should undergo the following ventilator management strategies to decrease mortality and morbidity: LPSs to include targeting low tidal volume ventilation (LTVV) [tidal volume (VT) 4–8 mL/kg ideal body weight (IDBW), Pplat ≤30 cmH2O and ∆P ≤15] (4-6). Despite adhering to these guidelines VILI still may occur, resulting in increased mortality, secondary complications of MV, such as PTX, pneumomediastinum, pneumoperitoneum and subcutaneous emphysema (7,8).
Pneumomediastinum is defined as the abnormal presence gas within the mediastinum. This phenomenon was first described by Laennec et al. in 1819 and the occurrence of a spontaneous pneumomediastinum by Hamman in 1939 (9). Macklin identified mechanism by which air can dissect by itself via artificial channels, along sheaths of the pulmonary vessels, leading to pneumomediastinum, as demonstrated in experiments in cats and other animals, labeled as the “Macklin effect” (10). Macklin’s research further identified that by overinflating alveoli, would cause small ruptures in the alveoli floor, leading to air dissecting along pulmonary vessels, leading to pulmonary interstitial emphysema into the mediastinum. Mechanistically, this would be similar to MV utilizing positive pressure, leading to VILI. Other reports of etiology of spontaneous pneumomediastinum, may be related to type I and II pneumocyte breakdown when infected by virus (11).
Reported incidence of barotrauma, defined as PTX, pneumomediastinum, pneumoperitoneum or subcutaneous emphysema, in mechanically ventilated ARDS patients can be as high as 2.9% (12). At our institution, our rate of barotrauma in C-ARDS is 1.3%, with a mortality of 75% among this small cohort, suggesting pneumomediastinum may be a predictor of poor outcome.
Risk of developing pneumomediastinum while mechanically ventilated has been primarily related to risk factors for barotrauma and high airway pressures (12). All of our patients had elevated airway pressures and ∆Ps, which could have led to development of pneumomediastinum. Although this was mitigated by limiting pressures via PRVC, APRV or AC, one would expect that as compliance worsens, the risk of barotrauma increases, as regional lung tissue is exposed to ventilator forces, in non-dependent and dependent areas at differing phases of ventilation (13). Further review of COVID-19 therapies including steroids, has not shown to contribute to increased risk of VILI, in context of wide use of steroids, since results of the RECOVERY trial report benefit (14,15).
Management of pneumomediastinum can vary, depending on whether hemodynamic embarrassment is occurring, or worsening ventilation or oxygenation is present. For initial evaluation, CXR is diagnostic for extensive pneumomediastinum, but may only identify 75% of cases, whereas CT identifies 100% of cases, often limited by stability of patient to leave intensive care unit (ICU) (16). Once pneumomediastinum is identified, determination of cause, as related to PTX, upper airway injury, tracheobronchial disruption, or esophageal injury exists. If only pneumomediastinum without above complicating features, the clinician may opt to invoke continued conservative therapy, by limiting PEEP as able, utilizing LPS and targeting Pplat ≤30 cmH2O and ∆P <15.
If conservative therapy fails and inability to ventilate patient or hypotension develops, due to extensive subcutaneous pneumomediastinum and potentially tension pneumomediastinum physiology, consultation of cardiothoracic surgeon may be warranted, to perform “gills” procedure (17). “Gills” procedure may improve ability to ventilate, by relieving tension pneumomediastinum and relieve tension pneumopericardium.
Our four patients were managed conservatively, with LPS, targeting Pplat ≤30 cmH2O, ∆P <15 cmH2O and lower PEEP, as tolerated. Only in 1 patient was a possible source of pneumomediastinum identified, which was thought to be related to the endotracheal tube manipulation, all other patients were likely related to combination of barotrauma, breakdown of type I and II pneumocytes and the Macklin effect.
Subcutaneous and mediastinal emphysema is an uncommon finding associated with C-ARDS and is likely related to the combination of barotrauma the “Macklin effect” and breakdown of type I and II pneumocytes related to COVID-19 infection. Conservative management of subcutaneous and mediastinal emphysema is usually all that is required, unless tension pneumomediastinum occurs, or PTX is present.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jeccm-20-149
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jeccm-20-149). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). The Institutional Review Board of Washington Hospital Center of Washington DC, approved this study and waived the need for informed consent from individual patients owing to the retrospective nature of the study (IRB ID: STUDY0003052). Additionally, all patients are deceased, and all exhaustive attempts have been made to contact the family and they were not accessible. Any potential patient’s identifiers have been removed from the description/images and this paper has been anonymized not to cause any harm to the patient or the family. We believe our case would be a great teaching case for the clinicians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen L, Xia HF, Shang Y, et al. Molecular mechanisms of ventilator-induced lung injury. Chin Med J (Engl) 2018;131:1225-31. [Crossref] [PubMed]
- Tonetti T, Vasques F, Rapetti F, et al. Driving pressure and mechanical power: new targets for VILI prevention. Ann Transl Med 2017;5:286. [Crossref] [PubMed]
- Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020;117:10970-5. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315:788-800. Erratum in: JAMA 2016;316:350. [Crossref] [PubMed]
- Guérin C, Papazian L, Reignier J, et al. Effect of driving pressure on mortality in ARDS patients during lung protective mechanical ventilation in two randomized controlled trials. Crit Care 2016;20:384. [Crossref] [PubMed]
- Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Pingleton SK. Barotrauma in acute lung injury: is it important? Crit Care Med 1995;23:223-4. [Crossref] [PubMed]
- Maunder RJ, Pierson DJ, Hudson LD. Subcutaneous and mediastinal emphysema. Pathophysiology, diagnosis, and management. Arch Intern Med 1984;144:1447-53. [Crossref] [PubMed]
- Gasser CR, Pellaton R, Rochat CP. Pediatric spontaneous pneumomediastinum: narrative literature review. Pediatr Emerg Care 2017;33:370-4. [Crossref] [PubMed]
- Macklin MT, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: an interpretation of the clinical literature in the light of laboratory experiment. Medicine 1944;23:281-358. [Crossref]
- Gralinski LE, Baric RS. Molecular pathology of emerging coronavirus infections. J Pathol 2015;235:185-95. [Crossref] [PubMed]
- Anzueto A, Frutos-Vivar F, Esteban A, et al. Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive Care Med 2004;30:612-9. [Crossref] [PubMed]
- Carrasco Loza R, Villamizar Rodríguez G, Medel Fernández N. Ventilator-induced lung injury (VILI) in acute respiratory distress syndrome (ARDS): volutrauma and molecular effects. Open Respir Med J 2015;9:112-9. [Crossref] [PubMed]
- Umegaki T, Sakamoto S, Nishi K, et al. Impact of steroid medication before hospital admission on barotrauma in mechanically ventilated patients with acute respiratory distress syndrome in intensive care units. J Anesth 2014;28:681-6. [Crossref] [PubMed]
- Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693-704. [Crossref] [PubMed]
- Okada M, Adachi H, Shibuya Y, et al. Diagnosis and treatment of patients with spontaneous pneumomediastinum. Respir Investig 2014;52:36-40. [Crossref] [PubMed]
- Kiefer MV, Feeney CM. Management of subcutaneous emphysema with "gills": case report and review of the literature. J Emerg Med 2013;45:666-9. [Crossref] [PubMed]
Cite this article as: Clark PA, Yohannes S, Pratt A. Subcutaneous and mediastinal emphysema, uncommon complications of COVID-19 ARDS: a case series. J Emerg Crit Care Med 2021;5:26.


