
Case report: challenges in making the diagnosis of sporadic Creutzfeldt-Jakob disease
Introduction
Creutzfeldt-Jakob disease (CJD) is the most common human prion disease and leads a rapidly progressive clinical course without any definitive cure. Its current classification includes sporadic, genetic, iatrogenic and variant. Its rare occurrence, variable presentations, limited testing sites, and difficulties in obtaining tissue diagnosis attribute to its challenges in diagnosis (1-4). Sporadic CJD (sCJD) affects approximately one in one million individuals worldwide and is generally regarded as a spontaneous neurodegenerative disorder (5,6). It has a reported onset of 62 years of age and is more common in Caucasian population (6). The initial presentations include variable neurological and psychiatric manifestations that often mimic other common disease entities. Subsequently, a neurological change may occur which requires further comprehensive workup that involves neurological imaging, electroencephalogram (EEG), and cerebrospinal fluid (CSF) analysis. We present a rare case of sCJD that requires several hospitalizations from symptoms onset prior to finally being admitted to the intensive care unit (ICU) for concerns of sCJD. We faced multiple challenges in making his diagnosis: nonspecific symptoms, presence of a pacemaker, and multiple rejected CSF specimen. When we finally performed magnetic resonance imaging (MRI), initial report did not comment on characteristic findings of sCJD.
We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jeccm-20-180).
Case presentation
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent could not be obtained from the deceased patient’s next-of-kin for publication of this case report and accompanying images despite all reasonable attempts. Every effort has been made to protect the patient’s identity and there is no reason to believe that our patient would have objected to publication.
Our patient was a 63-year-old right handed Caucasian male, who was otherwise in good health and living independently with his two dogs. He had a past medical history of hypertension, gastroesophageal reflux disease, and cervical spinal stenosis. He reported no significant family history and had appendectomy as a child. He denied using tobacco or illegal drugs, but he drank socially in moderate amount. He was a retired correctional department officer. His most recent travel before his first admission to the hospital was 6 months prior to San Diego for fishing.
The patient initially presented to our hospital for 5-week onset of visual changes along with other generalized complaints of neck pain, headache, lightheadedness, 15-pound unintentional weight loss, increase in temperature sensitivity, numbness and tingling in his left foot. On his physical exam, he exhibited slight slurring of speech, lack of concentration, failed finger-to-nose test, but his cranial nerves II-XII were intact. His computed tomography (CT) of the brain without contrast did not show any acute abnormalities. He was admitted to the ward to monitor any acute neurological changes. He was diagnosed with binocular horizontal diplopia and discharged to follow-up with an ophthalmologist for his symptoms.
Before he could make his appointment, he was re-admitted for palpitation, shortness breathing and dizziness. He developed atrial fibrillation with rapid ventricular response from prior sinus bradycardia. He failed medical treatment and eventually required the placement of a dual-chamber MRI-compatible permanent pacemaker. The patient was started on apixaban on post-op day 2 for stroke prophylaxis. However, on the following day, his nurse called a Stroke Alert for sudden onset of aphasia. The patient underwent CTs stroke protocol which showed no intracranial hemorrhage, large vessel occlusion, infarction, or ischemic penumbra. His symptoms nearly resolved in the ICU with residual left visual field neglect. After 24 hours of neurological monitoring, he was transferred to a medical ward. He was eventually referred to inpatient rehabilitation due to significant functional decline noted by his physical therapist.
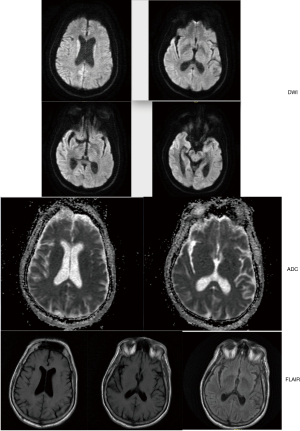
Our patient only stayed at his rehabilitation facility for less than 24 hours before needing to be transferred back for subacute encephalopathy and homicidal ideation. When he first arrived at the rehabilitation unit, he experienced significant difficulties with wheelchair transfer, intermittent confusion and ataxia. Then he attempted to strike two staff members, exhibited visual hallucination and violent outrage. He was re-admitted to our acute care hospital to workup his acutely altered mental status and placed on legal hold. Neurology team noted his new neurological changes of myolonus, dysarthria, and the lack of visual perception except for light and motion. His CT brain showed generalized cerebral and cerebellar atrophic changes and left-sided sphenoid sinusitis. MRI (Figure 1) reports mild cerebral volume loss without acute intracranial pathology. We obtained EEG (Figure 2A) that recorded periodic sharp wave complexes (PSWC) and triphasic waves. These findings were consistent with moderate encephalopathy, and PSWC raised concerns for possible prion disease. His CSF findings showed hazy and red in appearance, white blood cell (WBC) 7 µL, red blood cell (RBC) 2,000 µL, polynuclear WBCs 65%, lymphocytes 22%, monocytes 13%, total protein 68 mg/dL, and glucose of 68 mg/dL. His serum WBC was 12.5×10^3/mm3 and the rest of his labs, including basic metabolic panel, liver function test, thyroids studies, and ammonia were within normal limits. Blood culture and urine culture were no growth to date. His CSF was sent for comprehensive infectious investigations including 14-3-3.
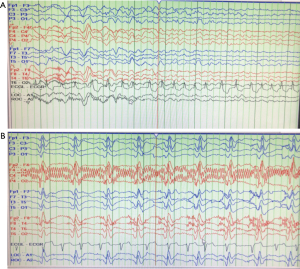
We started empiric treatment for various infectious diseases including ceftriaxone, fluconazole, doxycycline, and ampicillin. We initiated levetiracetam twice a day for seizure prophylaxis and used benzodiazepines sparingly for severe myoclonus. For one week, we used high-dose methylprednisolone and intravenous immunoglobulin to treat possible autoimmune encephalitis. Despite these treatments, our patient’s mental status and overall health continued to deteriorate daily. His repeated EEG showed characteristic Bilateral Synchronous Periodic Epileptiform Discharges (BiPEDs) (Figure 2B), suggesting sCJD. Three days later, he required endotracheal intubation for respiratory failure due to E. Coli pneumonia, and he was started on piperacillin-tazobactam. Radiology recommended delaying further MRI for at least 6 weeks from his pacemaker placement so that the leads can epithelialize. We had performed multiple lumbar punctures, as outside laboratory rejected our CSF specimens for testing 14-3-3. Eventually, we proceeded with withdrawing of futile medical care according family wishes, after confirmation on our CSF testing came back positive for 14-3-3 protein. His family did not request a confirmatory autopsy.
Discussion
SCJD occurs in 1 in 1,000,000 individuals worldwide with mean onset of 62 years of age and slight Caucasian predominance (6). In emergency settings such as initial visits to emergency department or acute decompensation within hospital, sJCD is difficult to diagnose due to its heterogenous clinical presentations. Two cardinal features are behavioral abnormalities and myoclonus in the setting of rapidly developing dementia (6). The pathological basis is thought to be the result of abnormal infectious prion proteins replacing normal ones over time leading to neurotoxicity (6,7). Therefore, the definitive diagnosis is established by standard neuropathological techniques with confirmation of protease-resistant PrP and/or presence of scrapie-associated fibrils (8). The Center for Disease Control and Prevention (CDC) outlines two diagnostic pathways for probable sCJD. The first one includes neuropsychiatric disorder plus positive RT-QuIC in CSF or other tissues (8). The second pathway requires three components: symptoms, supportive diagnostic studies, and exclusion of alternative diagnoses. The symptoms include rapidly progressive dementia and two findings of myoclonus, visual/cerebellar signs, pyramidal/extrapyramidal signs, and akinetic mutism. Studying findings include typical EEG features, 14-3-3 protein in CSF assay and MRI results (8).
Our patient received probable diagnosis using the latter pathway, and we faced many diagnostic challenges during his complicated clinical course. First, our patient presented with visual changes that were nonspecific and did not require further inpatient workup. His subsequent stroke-like symptoms alarmed us to focus on his modifying risk factor of newly diagnosed atrial fibrillation. His bizarre psychiatric manifestations of homicidal ideation and visual hallucination at inpatient rehabilitation facility further complicated in narrowing down his medical differential diagnoses. Eventually, during his final re-admission to the ICU, his constellations of symptoms over the previous month became highly suggestive of sCJD. Literature reported similar challenges in arriving at this diagnosis due to low awareness of sCJD and its similarities to other clinical disease entities (2,4-7). These patients often required multiple visits to emergency department resulting in admissions for further inpatient workup. After excluding possible diagnoses of autoimmune, infectious, malignant, and toxic-metabolic etiologies, sCJD should be considered in the differential diagnosis when rapid dementia accompanies myoclonus and gait disturbances.
MRI, EEG, and CSF studies are common diagnostic modalities to help to include and exclude wide range of differential diagnoses of neurological diseases. MRI is the most useful in the diagnosis of CJD and maybe the most sensitive test during early stages of disease, with 83% to 92% sensitivity, and 87% to 95% specificity (9). It has characteristic findings of hyperintense signal on diffusion weighted imaging (DWI), fluid attenuated inversion recovery (FLAIR) sequence and T2-weighted images, most commonly in the area of basal ganglia and thalamic area and in a cortical ribboning pattern of cortex. Other reported areas also included frontal, parietal, visual, temporal, limbic and hippocampal cortices (1). In our patient, he recently had MRI-compatible pacemaker placed for his atrial fibrillation. After his initial MRI exam that showed no acute findings, radiology department alerted us to postpone further MRIs to allow his pacemaker endothelialize. While our medical treatment team followed the radiologist’s reading results and recommendations, I noticed at the time of this case report that the patient’s MRI did exhibit characteristic findings of sCJD: cortical ribbon pattern (10). Figure 1 demonstrated signal hyperintensity on DWI involving right parietal and occipital lobes, but its FLAIR sequence did not show any abnormalities.
Our patient’s second supportive study was EEG. There were two obtained, four weeks apart, during his multiple hospitalizations. The first one (Figure 2A) showed PSWC and triphasic waves. Our neurologist diagnosed him with moderate encephalopathy and probable concerns for prion disease. His repeated EEG demonstrated characteristic Bilateral Synchronous Periodic Epileptiform Discharges (BiPEDs) (Figure 2B), and he was shortly intubated tracheally due to inability to protect his airway. Even though EEG is not as sensitive as MRI or CSF analysis when used to diagnose sCJD, it can be used as a supporting method (8), with 65% sensitivity and 90% specificity (6). False-positive EEG results have been reported in Alzheimer’s disease and vascular dementia, and positive results may not be recorded in the initial stages of the illness (6).
The third diagnostic exam was our patient’s CSF assay. His family expressed wishes to wait for CSF confirmation of either 14-3-3 protein or RT-QuIC results prior to making medical decisions based on probable diagnosis of sCJD. RT-QuIC in CSF, tau, real-time quaking-induced conversion, is the most sensitive and specific CSF diagnostic test for sCJD (6). 14-3-3 in CSF is non-specific test finding, and has an overall 92% sensitivity and 80% specificity in one systematic review (11). Diseases of false positives include Alzheimer’s disease, vascular dementia, metabolic and viral encephalopathies, and paraneoplastic syndromes (6). Blood contaminations and hemolysis of RBC would significantly release 14-3-3 protein after 2 days of room temperature (8,12). For our patient, we received multiple rejections from laboratory due to unfrozen sample passed thirty-minutes of collection time and blood contamination. The patient also had very low quantity in CSF sampling each time when we performed lumbar punctures, which made serial collections days apart very difficult as well. Waiting for these test results to come back from specialized centers added more time in delay to confirm our clinical diagnosis of sCJD.
In conclusion, we presented diagnostic challenges in a rare case of sCJD which progressed to death within 3 months of symptoms onset. We followed the outline of CDC diagnostic criteria for probable sCJD after other differential diagnoses were excluded. Our patient had key clinical features of rapid progressive dementia, myoclonus and visual and cerebellar disturbances. His EEGs progressed from PSWCs to BiPEDs, and his CSF received final confirmation of 14-3-3 protein after several attempts. We also noted characteristic MRI findings of cortical ribboning pattern after the final case review. As a scientific community, we need to raise disease awareness of sCJD and provide families with social and educational support. Even though there is no effective treatment of sCJD, arriving at the diagnosis quickly would help patients to enroll in disease research and allow families to have final closure.
Acknowledgments
The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
Funding: This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity.
Footnote
Reporting Checklist: The author has completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jeccm-20-180
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jeccm-20-180). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent could not be obtained from the deceased patient’s next-of-kin for publication of this case report and accompanying images despite all reasonable attempts. Every effort has been made to protect the patient’s identity and there is no reason to believe that our patient would have objected to publication.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Manix M, Kalakoti P, Henry M, et al. Creutzfeldt-Jakob disease: updated diagnostic criteria, treatment algorithms, and the utility of brain biopsy. Neurosurg Focus 2015;39:E2 [Crossref] [PubMed]
- Mugilan SR, Joseph JP. A case of probable sporadic Creutzfeldt-Jacob Disease in a tertiary care hospital in Malaysia. Med J Malaysia 2018;73:433-5. [PubMed]
- Dorey A, Tholance Y, Vighetto A, et al. Association of cerebrospinal fluid prion protein level and distinction between Alzheimer disease and Creutzfeldt-Jakob disease. JAMA Neurol 2015;72:267-75. [Crossref] [PubMed]
- Uttley L, Carroll C, Wong R, et al. Creutzfeldt-Jakob disease: a systematic review of global incidence, prevalence, infectivity, and intubation. Lancet Infect Dis 2020;20:e2-e10. [Crossref] [PubMed]
- Zerr I, Parchi P. Chapter 9: Sporadic Creutzfeldt-Jakob disease. Handbook of Clinical Neurology 2018;153:155-74. [Crossref] [PubMed]
- Appleby BS, Cohen ML. Creutzfeldt-Jakob disease, UptoDate, 2020. Available online: https://uptodate.medcity.net/contents/creutzfeldt-jakob-disease?search=cjd&source=search_result&selectedTitle=1~86&usage_type=default&display_rank=1
- Zerr I, Hermann P. Diagnostic challenges in rapidly progressive dementia. Expert Rev Neurother 2018;18:761-72. [Crossref] [PubMed]
- Center of Disease Control and Prevention. CDC’s Diagnostic Criteria for Creutzfeldt-Jakob disease (CJD), 2018. Available online: https://www.cdc.gov/prions/cjd/diagnostic-criteria.html
- Macfarlane RG, Wroe SJ, Collinge J, et al. Neuroimaging findings in human prion disease. J Neurol Neurosurg Psychiatry 2007;78:664. [Crossref] [PubMed]
- Abdulmassih R, Min Z. An ominous radiographic feature: cortical ribbon sign. Intern Emerg Med 2016;11:281-3. [Crossref] [PubMed]
- Muayqil T, Gronseth G, Camicioli R. Evidence-based guideline: diagnostic accuracy of CSF 14-3-3 protein in sporadic Creutzfeldt-Jakob disease: report of the guideline development subcommittee of the American Academy of Neurology. Neurology 2012;79:1499. [Crossref] [PubMed]
- Schmitz M, Ebert E, Stoeck K, et al. Validation of 14-3-3 protein as a marker in Sporadic Creutzfeldt-Jakob disease Diagnostic. Mol Neurobiol 2016;53:2189-99. [Crossref] [PubMed]
Cite this article as: McWhorter Y. Case report: challenges in making the diagnosis of sporadic Creutzfeldt-Jakob disease. J Emerg Crit Care Med 2021;5:39.


