
Pathophysiological determinants of arterial carbon dioxide tension (PaCO2) in spontaneously breathing and mechanically ventilated patients
Changes in PaCO2 in hospitalised patients are common and associated with an increased risk of morbidity and mortality. Although many clinicians are aware of the physiological mechanisms for PaCO2 homeostasis, they often have difficulty understanding how different compensatory mechanisms interact, and why such interactions are not always successful in achieving normocapnia. Incorrect interpretation of PaCO2 level—even when it is within the normal range—can have dangerous consequences in a spontaneously breathing patient (1). In this correspondence, we briefly describe how we can visually interpret the interactions of different pathophysiological mechanisms in determining PaCO2 in a spontaneously breathing or mechanically ventilated patient.
In a spontaneously breathing patient, there are two determinants of PaCO2. The respiratory drive from the brain is an active system (which can increase minute ventilation up to 10 L/min for every 3 mmHg PaCO2 increment unless PaCO2 is exceedingly high) (1); whilst the mathematical relationship between alveolar CO2 tension (or PaCO2 for simplicity), carbon dioxide production (VCO2 ~200 mL/min for an average adult that can increase up to 10 folds with vigorous exercise) and minute alveolar ventilation represents a passive system (Figure 1A) (2). Minute alveolar ventilation is equal to the minute ventilation minus the wasted ventilation due to the physiological dead space which is the sum of anatomical and alveolar dead space. The interaction between the active and passive systems defines the PaCO2.
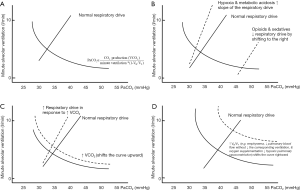
An increase in respiratory drive due to hypoxia or metabolic acidosis will increase the ‘slope’ of the active respiratory drive system, resulting in an increase in minute ventilation which will reduce PaCO2. As such, a PaCO2 within the normal range is actually abnormal in the presence of significant metabolic acidosis, and would signify concomitant respiratory drive depression (1). Respiratory depression due to opioids and sedatives will shift the active respiratory drive system to the right (Figure 1B), resulting in a lower minute ventilation and a higher PaCO2. An increase in VCO2 will shift the passive system upward, resulting in a higher PaCO2, until the active respiratory drive system shifts the slope upward to normalise the PaCO2 (Figure 1C).
An increase in alveolar dead space—which can occur due to emphysema, reduced pulmonary blood flow without a corresponding reduction in ventilation or over-ventilating poorly perfused alveoli [i.e., ↑ overall ventilation to perfusion (V/Q) ratio], ↑ V/Q heterogeneity in acute respiratory distress syndrome (ARDS) and pneumonia (3), or attenuation of the normal hypoxic pulmonary vasoconstriction due to oxygen supplementation)—will shift the passive system to the right, resulting in a higher PaCO2 (Figure 1D). Acute pulmonary embolism would theoretically increase alveolar dead space; an elevation of PaCO2 is, however, often not observed. This is because any increase in PaCO2 and reduction in arterial oxygen tension (PaO2) will be sensed by the medullary and carotid body chemoreceptors, respectively, which will increase the respiratory drive to increase minute ventilation, thereby lowering PaCO2. In fact, ‘overcompensation’ resulting in respiratory alkalosis in acute pulmonary embolism due to reflex stimulation of irritant and juxta capillary sensors in the lung is common (4). As for a patient with emphysema, administrating excessive oxygen can increase the patient’s alveolar dead space and aggravate any existing hypercapnia by abolishing the hypoxic pulmonary vasoconstriction. Due to an overinflated chest cavity and a flattened diaphragm, patients with emphysema will have a limited capacity to increase their minute ventilation to normalise their PaCO2. Furthermore, oxyhaemoglobin has a relatively linear and also lower CO2 binding capacity than deoxyhaemoglobin. Increasing PaO2 with excessive supplemental oxygen can further aggravate hypercapnia through the Haldane effect (2).
Understanding the pathophysiological determinants of PaCO2 also has utility for patients who are mechanically ventilated. Under such circumstances, the active respiratory drive system is replaced by the setting on the ventilator and the passive system affects the PaCO2 level through its interactions with the ventilator. Increasing ventilating rate or tidal volume excessively in a patient with emphysema can induce dynamic hyperinflation which can increase hypercapnia by increasing alveolar dead space, in addition to creating a disadvantage in the respiratory mechanics disallowing any spontaneous breaths (2). In patients with ARDS, excessive positive-end-expiratory-pressure (PEEP) can over-distend alveoli that are already well-ventilated, increasing alveolar dead space and hypercapnia (3). In judging whether a patient is ready for weaning off from a ventilator, a high PaCO2 (>45 mmHg) despite a high minute ventilation (>10 l/min) suggests that there is a substantial elevation in alveolar dead space. In this situation, weaning is unlikely to be successful until an improvement in the underlying lung condition (e.g., ARDS and its associated increased V/Q heterogeneity) has occurred—which means more time on the ventilator is needed (1).
In summary, understanding how the pathophysiological determinants of PaCO2 is useful in the appropriate interpretation of PaCO2 and hence also the treatment of patients with type II respiratory failure.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jeccm-21-7). Dr. KMH serves as an unpaid editorial board member of Journal of the Emergency and Critical Care Medicine from May 2017 to April 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tobin MJ. Why Physiology Is Critical to the Practice of Medicine: A 40-year Personal Perspective. Clin Chest Med 2019;40:243-57. [Crossref] [PubMed]
- Tobin MJ, Laghi F, Jubran A. Ventilatory failure, ventilator support, and ventilator weaning. Compr Physiol 2012;2:2871-921. [Crossref] [PubMed]
- Robertson HT. Dead space: the physiology of wasted ventilation. Eur Respir J 2015;45:1704-16. [Crossref] [PubMed]
- Goldhaber SZ, Elliott CG. Acute pulmonary embolism: part I: epidemiology, pathophysiology, and diagnosis. Circulation 2003;108:2726-9. [Crossref] [PubMed]
Cite this article as: John S, Ozanne R, HO KM. Pathophysiological determinants of arterial carbon dioxide tension (PaCO2) in spontaneously breathing and mechanically ventilated patients. J Emerg Crit Care Med 2021;5:30.


