
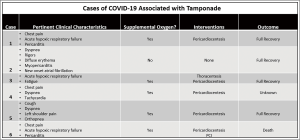
How COVID broke my heart: a case report of tamponade after SARS-CoV-2 infection
Introduction
A patient arriving by emergency medical services with complaints of chest pain radiating to the back, shortness of breath, and hypertension after the diagnosis of COVID-19 warrants a cardiac workup including electrocardiogram (EKG), troponin, and a computed tomography angiogram (CTA) of the chest and abdomen as thromboembolism is a known effect of COVID-19 infection (1). We present a clinical case where the patient was diagnosed with COVID-19 17 days prior, but was never hospitalized, and later presented with an acute STEMI complicated by a post-viral pericardial effusion which resulted in an emergent PCI with multiple stents, complicated by intraoperative stent thrombosis, and admission to the intensive care unit requiring vasopressor support and emergency pericardiocentesis after the development of cardiac tamponade physiology. To our knowledge, only six cases of COVID-19 associated pericardial effusions have been reported and four cases resulted in cardiac tamponade (2-5). This case is unique because the patient developed an STEMI associated with the pericardial effusion, developed intraoperative stent thrombosis, and was on long-term anticoagulation with apixaban for paroxysmal atrial fibrillation. This case highlights both the prothrombotic state associated with COVID-19 infections as well as the complications of post-viral pericarditis leading to effusion. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jeccm-21-11).
Case presentation
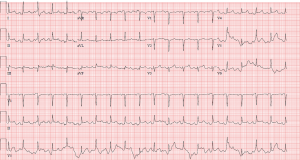
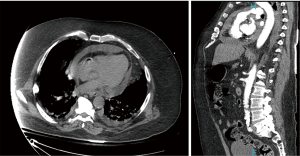
Patient is a 67-year-old Caucasian male with past medical history of type II diabetes mellitus, paroxysmal atrial fibrillation on apixaban, essential hypertension, left internal carotid aneurysm, and diagnosis of COVID-19 pneumonia approximately 3 weeks prior presented with acute onset shortness of breath, chest pain radiating to the back, and hypertension. He had no relevant family medical history of heart disease. Initial laboratory results showed lactic acid of 15.5 mmol/L, high sensitivity troponin 68 pg/mL, sodium 126 mmol/L, and potassium 5.9 mmol/L. Inflammatory and clotting markers significant for CRP of 14.3, d-dimer of 1.88, ferritin 780.7, and LDH of 536. On physical exam, the patient had an irregularly irregular heart rate with muffled heart sounds, coarse breath sound with rhonchi bilaterally, and cold extremities. An EKG en route (Figure 1) showed inferolateral STEMI and plan was made for emergent PCI, but due to the hypertension and tearing back pain, patient first underwent a CTA (Figure 2), which showed a moderate pericardial effusion, no pulmonary embolism or aortic dissection and patient underwent emergency PCI.
PCI showed an ejection fraction of 40% with inferior hypokinesis, a normal left main coronary artery, a 95% occluded left circumflex coronary artery (LCX), and a 90% occlusion of the right coronary artery (RCA). During the catheterization, the patient developed atrial fibrillation with rapid ventricular rate and marked hypotension and required direct current cardioversion which failed twice. Amiodarone was started and patient went into pulseless electrical activity (PEA). Cardiopulmonary resuscitation (CPR) was initiated and after two rounds of CPR, epinephrine, and endotracheal intubation. Return of spontaneous circulation (ROSC) was achieved and two drug eluting stents (DES) were placed to the LCX and RCA. The PCI was complicated by an acute clot of the RCA stent requiring additional antiplatelet therapy. At this time, due to severe hypotension, the patient was started on norepinephrine. Post stenting, the patient again went into PEA arrest and after CPR, ROSC was achieved. A Swan-Ganz catheter was placed and the procedure completed.
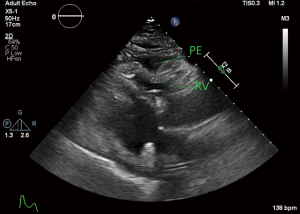
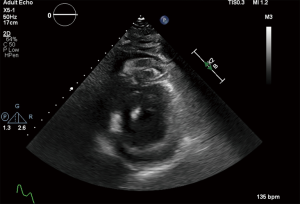
The patient was moved to the ICU with worsening hypotension, so vasopressin was started as a temporizing measure until definitive treatment. Laboratory results at this time showed potassium of 7.0 mmol/L, creatinine 2.4 mg/dL, glucose of 763 mg/dL, and lactic acid of >20 mmol/L. Postintubation arterial blood gas (ABG) showed pH of 7.11, pCO2 of 28.7 mmHg, and a pO2 of 134 mmHg. Repeat ABG worsened at pH of 6.91, pCO2 35 mmHg, and pO2 of 233. A bicarbonate infusion was started at this time and a stat transthoracic echocardiogram showed a moderate circumferential pericardial effusion with evidence of tamponade physiology including right atrial collapse and significant variation in mitral valve inflow pattern (Figures 3-5). Due to the worsening hypotension, cardiogenic shock, and need for the addition of phenylephrine and dobutamine, the decision was made with the family to proceed with bedside pericardiocentesis in an attempt to reduce the pressure within the pericardium.
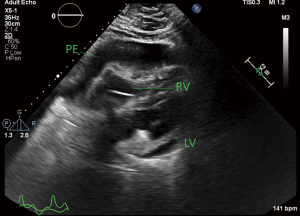
At the time of the pericardiocentesis, patient was on maximal doses of four vasopressors including norepinephrine, vasopressin, phenylephrine, and dobutamine with a mean arterial pressure (MAP) of 30 on arterial line tracing. Central venous pressure was 16 mmHg, cardiac index was 2.4 L/min/m2, and cardiac output was 5.3 L/min. Pericardiocentesis was performed with initial drainage of 295 mL of cloudy, straw colored fluid. After removal of this fluid, MAP increased to 72. Echocardiogram after pericardiocentesis showed a small residual pericardial effusion with no diastolic collapse and support catheter in the right ventricle. Patient remained critical, but stabilized long enough to start a bicarbonate infusion for his acidosis, but shock worsened and attempted drainage of residual pericardial effusion by retained catheter did not alleviate the refractory shock. Despite the aggressive measures initially undertaken, the patient failed to progress and with worsening cardiogenic shock, lactic acidosis, hyperglycemia, and ventilator requirements, the decision was made by the family to pursue comfort measures and the patient passed.
After the patient passed, the results of the pericardial effusion fluid samples showed a total protein level of 4.7 mg/dL, lactate dehydrogenase (LDH) of 3,681 U/L, total nucleated cell count of 18.055 K/uL, RBC of 0.025 M/uL, and culture grew few staphylococcus aureus. No viral polymerase chain reaction (PCR) was performed. Pericardial fluid had the characteristics of an exudative process which further supports the diagnosis of a post-viral pericardial effusion due to COVID-19 infection. Post-myocardial infarction syndrome was also on the differential due to the exudative process, but due to chest pain beginning on the day of admission, stent clotting in PCI, and the continued elevation of inflammatory markers, this was thought to be less likely.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
COVID-19 has brought about a plethora of new concerns in critically ill patients, not the least of which is thromboembolic events. Despite COVID-19 having a predominantly respiratory effect, there have been reported complications involving many other organ systems and this case highlights the lasting effects of COVID-19 on the body by showing a prothrombotic state as well as the post-viral pericarditis leading to effusion. The rate of thromboembolic events was studied in one Italian hospital with 21% of patients with laboratory confirmed COVID-19 having thromboembolic events and half of that within the first 24 hours of hospital admission (6). This is further evidence that there are extrapulmonary complications of COVID-19 that can manifest themselves as early as 24 hours into admission.
The development of myocarditis in COVID-19 patients (Figure 6) happens much the same way that any viral myocarditis occurs. In Europe, approximately 25% of hospitalized COVID-19 patients have some evidence of acute myocardial damage, which is seen with elevated troponin levels (7). Unfortunately, there is little known about the mechanism of COVID-19 related myocarditis but the leading theories suggest there is a downregulation of angiotensin converting enzyme 2 (ACE2) expression with massive inflammation which causes the myocarditis or there is a local immune response that causes release of cytokines and chemokines which recruits monocytes and T-cells to cause an inflammatory response (7-9). Another theory is that there is increased fluid in the pericardial sac due to decreased resorption in the setting of increased systemic vascular pressure because of the congestive heart failure or pulmonary hypertension.
With the pericardial fluid culture resulting in few staphylococcus aureus, it is likely that this was bacterial translocation. The patient was never admitted to the hospital after contracting COVID-19 and never had central venous access until this admission. With centrally placed venous catheters at the superior vena cava and right atrium junction, there is known to be an area of irritation from the catheter which allows bacteria to enter the pericardial space, especially after intravenous procedures such as PCI, arterial line placement, and central venous catheter placement.
In this case, the pericardial effusion was noted incidentally on computed tomography (CT) imaging. The management of pericardial effusions in previously diagnosed COVID-19 patients should be approached in the same way that other pericardial effusions are managed and the appropriate imaging to further delineate the size and effect on cardiovascular physiology is an echocardiogram. The pericardial effusion can be classified based on onset as acute (<1 week), subacute (>1 week but <3 weeks), and chronic (>3 weeks). It can also be classified based on size as mild (10 mm), moderate (10–20 mm), or large (>20 mm). The distribution can be described as loculated or circumferential. In normal pericardial sacs, there is between 10–50 mL of pericardial fluid, but in pericardial effusions there can be significantly more volume and it can be transudative, exudative, plasma ultrafiltrate, bloody, chylous pericardial fluid, purulent fluid, or air in the pericardium (10). To determine between the different etiologies of pericardial effusions, it is important to determine if it is transudative or exudative with serum and fluid protein and LDH, cytology for malignancy, biomarkers such as carcinoembryonic antigen (CEA) or cytoplasmic protein fragment of cytokeratin-21 (CYFRA-21), tuberculosis markers such as interferon gamma and adenosine deaminase, and microbiology for an infectious source.
The thromboembolic events that patients afflicted with COVID-19 have a predisposition for were, again, evident during the heart catheterization. Despite the patient being on long term anticoagulation with apixaban, receiving aspirin en route, and receiving periprocedural unfractionated heparin (antithrombin III enzyme inhibitor), aspirin, and ticagrelor (antiplatelet), the patient still developed a clot in the newly stented RCA DES and a tirofiban infusion was added (glycoprotein IIb/IIIa inhibitor).
Immediately after the heart catheterization, an echocardiogram was performed to assess the cardiac physiology as well as to better visualize the pericardial effusion seen on CT scan. Initial echocardiogram showed a moderate pericardial effusion with tamponade physiology. If, post catheterization, this was the first time that a pericardial effusion was noted on imaging, it should be assumed that this was a hemopericardium and cardiothoracic surgery should be consulted for emergency surgical treatment. In this case, since it was seen on imaging prior to catheterization, it was significantly less likely to be a hemopericardium with a critical bleed causing tamponade. As the patient was hemodynamically unstable with cardiac tamponade physiology, the next best approach is a bedside pericardiocentesis to attempt to drain some of the excess fluid and relieve the pressure in the pericardial sac. Any pericardial fluid drained should be sent to the laboratory for analysis, including specific gravity, protein, protein fluid/serum ratio, LDH, LDH fluid/serum ratio, glucose, blood cell counts, cultures, and viral PCR. If there is a concern for malignancy, cytology and tumor markers can also be obtained. The results of these studies would drive further management once stable enough to transfer from the ICU.
For patients who have a history of COVID-19 infection, long term effects are still not well understood, although it is certain that there is a predisposition to thrombotic events in these patients. It is important for clinicians to be aware of the prothrombotic events that are frequent in patients with COVID-19 history and can even happen in patients who are on long term anticoagulation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jeccm-21-11
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jeccm-21-11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shi Y, Wang G, Cai XP, et al. An overview of COVID-19. J Zhejiang Univ Sci B 2020;21:343-60. [Crossref] [PubMed]
- Fox K, Prokup JA, Butson K, et al. Acute Effusive Pericarditis: A Late Complication of COVID-19. Cureus 2020;12:e9074 [PubMed]
- Sauer F, Dagrenat C, Couppie P, et al. Pericardial effusion in patients with COVID-19: case series. Eur Heart J Case Rep 2020;4:1-7. [Crossref] [PubMed]
- Hua A, O'Gallagher K, Sado D, et al. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J 2020;41:2130. [Crossref] [PubMed]
- Dabbagh MF, Aurora L, D’Souza P, et al. Cardiac tamponade secondary to COVID-19. JACC Case Rep 2020;2:1326-30. [Crossref] [PubMed]
- Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9-14. [Crossref] [PubMed]
- Liu W, Liu Z, Li YC. COVID-19-related myocarditis and cholinergic anti-inflammatory pathways. Hellenic J Cardiol 2020; Epub ahead of print. [Crossref] [PubMed]
- Bavishi C, Bonow RO, Trivedi V, Abbott JD, Messerli FH, Bhatt DL. Special Article - Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog Cardiovasc Dis. 2020;63:682-9. [Crossref] [PubMed]
- Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz 2020;45:230-32. [Crossref] [PubMed]
- Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J. 2013;34:1186-97. [Crossref] [PubMed]
Cite this article as: Pietrangelo M, Hess J, Ellis L. How COVID broke my heart: a case report of tamponade after SARS-CoV-2 infection. J Emerg Crit Care Med 2021;5:27.


