Sepsis versus anaphylaxis—differentiating shock etiology: a case report involving Smoflipid
Introduction
Accurate diagnosis of the underlying shock etiology is essential to promptly provide the most appropriate treatment to prevent further clinical decompensation and end-organ failure. Shock is described as cellular and tissue hypoxia resulting from reduced oxygen delivery, increased oxygen consumption or utilization, and the mechanism of shock states are divided into four categories, hypovolemic, distributive, cardiogenic, and obstructive. The end-goal of shock management, i.e., restoration of sufficient systemic perfusion and a homeostatic balance of oxygen demand and supply, is ubiquitous between the four described shock states. However, the shock etiology would dictate the treatment modality to reach the goals mentioned above (1). Reliance on patient history, clinical features, diagnostic studies, and response to therapies are critical in the precise classification of shock, which allows the institution and continuation of appropriate management strategies.
In this case report, we discuss the unsuspecting etiology of an acute distributive shock state in a patient admitted to the hospital and subsequently transferred to the intensive care unit in acute shock following an intra-abdominal drain manipulation. A review of the patient’s complex course leading up to this presentation, the temporal relationship of events, diagnostics, and subsequent differential diagnosis and management will be presented. We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/jeccm-21-28).
Case presentation
A 53-year-old male with a history of drain placed in a duodenal perforation bed presented to the hospital, general care surgical service, following intra-abdominal drain manipulation and percutaneous cholecystectomy tube exchange in interventional radiology. His past medical history was significant for a retroperitoneal well-differentiated liposarcoma resection approximately three months before the index admission, which was complicated by duodenal perforation, which resulted in the need for re-operation and drain placement. Following drain placement, he started total parenteral nutrition (TPN) with Intralipid, which he continued at home without difficulty. His postoperative course was also complicated by ureteral obstruction secondary to a retroperitoneal fluid collection, which led to the need for a nephrostomy tube placement.
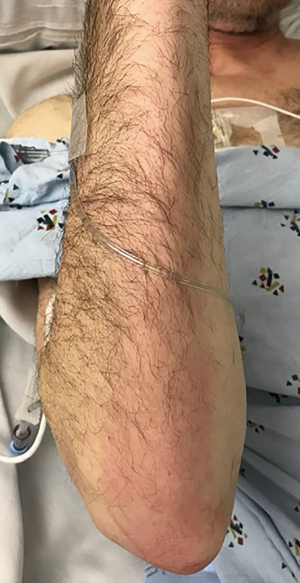
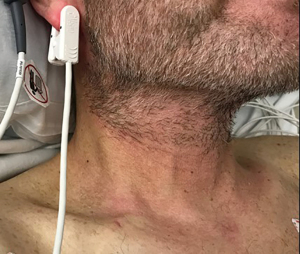
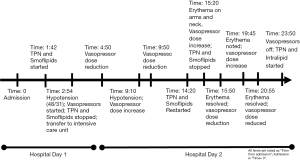
He developed profound acute hypotension on hospital day one, 48/31 [35] mmHg [systolic/diastolic (mean arterial pressure)], necessitating a rapid response activation. He was found to be unresponsive with fixed pupils. He was resuscitated with a total of four liters of crystalloid and one liter of colloid, transferred to the intensive care unit (ICU), started on norepinephrine and vasopressin infusions, given stress-dose hydrocortisone IV (50 mg every 6 hours). His TPN and lipids were stopped. He was also febrile and was treated with intravenous acetaminophen. Vasopressor doses were titrated down over the next two hours. Approximately four hours later, he developed pruritic erythema on both palms with associated hypotension requiring higher vasopressor doses. After one hour, his hypotension resolved, and he remained off vasopressors for approximately four hours. TPN and lipids (same bags from hospital admission) were resumed. About one hour later, he developed palmar erythema extending along the ulnar aspect up to the antecubital fossa bilaterally and on the neck (Figures 1,2). He was then found to have progressive hypotension, and vasopressors were re-initiated. TPN and lipids were stopped, and within 30 minutes, there was an improvement in hemodynamics and arm erythema resolved. He remained afebrile throughout the remainder of the hospitalization. Figure 3 outlines the timeline of events.
Diagnostic workup, including chest, abdomen, and pelvis CT scans, was unremarkable for shock etiology. Lactate was benign at 1.9 mmol/L (normal: 0.5–2.2 mmol/L), and tryptase was elevated at 23.8 ng/mL (normal: <11.5 ng/mL). A mild leukocytosis was noted at 12.6–109/L [normal: (3.4–9.6)×109/L], which was thought to be reactive in nature post-procedure. His other laboratory studies were unremarkable. Blood and urine cultures were obtained. The urine culture was positive for multi-drug resistant Escherichia coli (susceptible to piperacillin-tazobactam), which was also thought to be potentially contributing to his leukocytosis. Blood cultures were negative. He was empirically treated with piperacillin-tazobactam (3.375 grams every 6 hours) and vancomycin IV.
Given the temporal relationship of shock with TPN and lipids administration, along with tryptase elevation, allergy and immunology service was consulted for review. The patient did not report any history of food allergy. Further investigation revealed that the only difference in the TPN formulation used during his index hospitalization was the use of the lipid emulsion Smoflipid (Fat emulsion-soy-mct-oliv-fish oil) (Fresenius Kabi, Uppsala, Sweden) as opposed to Intralipid (Baxter Healthcare Corporation, Deerfield, Illinois, USA), which was used in his home TPN regimen. Subsequently, TPN with Intralipid was re-initiated on the evening of hospital day 2. The patient remained hemodynamically stable without the use of vasopressor support with no recurrence of pruritus or erythema. He was transferred to the general care surgical service on hospital day three and discharged in stable condition on hospital day four.
Approximately four weeks after hospital discharge, the patient underwent outpatient allergy evaluation. Skin prick testing to Smoflipid was negative, as was a total IgE and serum IgE testing to soy, olive, peanut, lobster, shrimp, crab, and salmon. Repeat tryptase was normal at 8.9 ng/mL. Given the lack of validation and poor predictive value of the skin testing performed and the severity of his clinical presentation with an elevated tryptase, strict avoidance of Smoflipid was recommended. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees, and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
Hypersensitivity to TPN is rare, and as such, no standardized guidelines exist regarding its evaluation and management. TPN formulations consist of multiple components to provide adequate nutritional support. A recent systematic review of 28 articles reporting 33 cases of PN hypersensitivity found that the most commonly identified causative allergens were the lipid emulsion (48.4%), multivitamin (33.3%), and amino acid solution (9%). Cutaneous symptoms were the most common clinical manifestation, occurring in 81.8% of patients. Anaphylaxis occurred in 45.4% of patients, and hemodynamic instability occurred in 21.2%. In the 17 patients who reacted to the lipid emulsion, ten were due to Intralipid, and only one was due to Smoflipid (unspecified or alternative agent implicated in the other six cases) (2). The presence or absence of pre-existing food allergies as a guide to TPN formulation selection is controversial. Intralipid contains egg phospholipid and soybean oil, while Smoflipid contains soybean, olive, and fish oils in addition to egg lecithin. While the package insert of Smoflipid lists pre-existing allergies to egg, soybean, fish, and peanut as contraindications to its use, Intralipid does not. Cases have been reported of reaction to Smoflipid in a patient with fish allergy and reaction to Intralipid in patients with previous egg and legume allergy (3-5). Interestingly, other medications known to contain soybean and egg lecithin, such as propofol, a commonly used anesthetic in emergency and critical care, have been deemed safe to use in egg and soy allergic patients by the national allergy associations (6). There is clearly a gap in knowledge surrounding the association of food allergy and PN reactions. In patients with PN reactions, basic principles include supportive care and the use of alternative agents if available. Consultation with an allergist is crucial to identifying the potential culprit and outline protocols for re-introduction if necessary. Additionally, proposed skin testing algorithms to help identify culprit agents are being developed (7).
Identification of anaphylaxis can be challenging and is typically based on clinical evaluation and the temporal relationships between the allergen and its resulting reaction. Measurement of tryptase can help provide objective evidence of mast cell involvement. While alpha and beta-tryptase can be released in an immature form from unstimulated mast cells, mature tryptases are released in actively degranulating mast cells along with histamine into the extracellular environment. This histamine release leads to the hemodynamic decompensation of anaphylactic shock; measurement of tryptase as it is released along with histamine can be a suitable objective surrogate marker for an anaphylactic reaction (8).
While the true etiology of this patient’s shock state cannot be singularly identified with full certainty, the temporality of shock in association with initiation, cessation, and re-initiation of Smoflipid (a new exposure) in combination with cutaneous symptoms and an elevated tryptase level supports anaphylaxis-like reaction as a large component of shock. Septic shock in the setting of drain manipulation may have contributed, but the time-series events and recurrence of shock after re-exposure to Smoflipid suggests that it played a major role.
This case highlights the importance of accurate identification of the etiology of shock state has in providing appropriate treatment. While the specific identification of shock state etiology may not always be possible or overtly straightforward, consideration of patient presentation, the temporality of hemodynamic instability, response to treatment, and other contributing factors (i.e., new medications or exposures) should be considered. Incorporation of all data points, both patient-provided and medical diagnostics along with clinical experience and prowess, coupled with expert consultation, should be synthesized to provide the patient an accurate diagnosis and appropriate treatment plan.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/jeccm-21-28
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jeccm-21-28). KBK serves as an unpaid editorial board member of Journal of Emergency and Critical Care Medicine from May 2020 to April 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of this work, ensuring that questions related to the accuracy or integrity of any part of this work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees, and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Standl T, Annecke T, Cascorbi I, et al. The Nomenclature, Definition and Distinction of Types of Shock. Dtsch Arztebl Int 2018;115:757-68. [Crossref] [PubMed]
- Christian VJ, Tallar M, Walia CLS, et al. Systematic Review of Hypersensitivity to Parenteral Nutrition. JPEN J Parenter Enteral Nutr 2018;42:1222-9. [Crossref] [PubMed]
- Hiyama DT, Griggs B, Mittman RJ, et al. Hypersensitivity following lipid emulsion infusion in an adult patient. JPEN J Parenter Enteral Nutr 1989;13:318-20. [Crossref] [PubMed]
- Lunn M, Fausnight T. Hypersensitivity to total parenteral nutrition fat-emulsion component in an egg-allergic child. Pediatrics 2011;128:e1025-8. [Crossref] [PubMed]
- Hernández CR, Ponce EC, Busquets FB, et al. Hypersensitivity reaction to components of parenteral nutrition in pediatrics. Nutrition 2016;32:1303-5. [Crossref] [PubMed]
- American Academy of Allergy Asthma & Immunology. Soy-allergic and egg-allergic patients can safely receive anesthesia. 2017. Available online: https://www.aaaai.org/Tools-for-the-Public/Conditions-Library/Allergies/soy-egg-anesthesia. Accessed January 21, 2021.
- Vu C, Quinn J, Reeves P. Anaphylaxis to Total Parenteral Nutrition: Developing an Approach to Diagnosis and Management. Annals of Allergy, Asthma & Immunology. (2021). Available online: https://apps.dtic.mil/sti/pdfs/AD1105199.pdf
- Schwartz, L. Laboratory tests to support the clinical diagnosis of anaphylaxis. Uptodate. (2020). Available online: https://www.uptodate.com/contents/laboratory-tests-to-support-the-clinical-diagnosis-of-anaphylaxis?search=tryptase§ionRank=1&usage_type=default&anchor=H4&source=machineLearning&selectedTitle=2~91&display_rank=2#H4
Cite this article as: Anderson R, Pitlick M, Perez A Jr, Kashani KB. Sepsis versus anaphylaxis—differentiating shock etiology: a case report involving Smoflipid. J Emerg Crit Care Med 2022;6:2.