
The effects of thiamine on patients with sepsis and septic shock
Introduction
Sepsis affects about 1.7 million people in the United States annually and results in over 270,000 deaths (1). Despite advances in critical care practices, sepsis remains the most common cause of death in non-cardiac intensive care units (ICUs). Sepsis manifests as decreased systemic vascular resistance resulting in decreased organ perfusion, and ultimately impaired oxygen delivery (2).
Patients who survive may suffer from residual organ dysfunction (3). Current management of sepsis focuses on administering intravenous (IV) fluids and vasopressors while treating the underlying infection and controlling the source of infection. Society of Critical Care Medicine (SCCM) guidelines do not address IV thiamine use in sepsis (4).
Thiamine has recently been used in sepsis as a potential adjunct to antibiotics, infection source control, and supportive care for patients with sepsis and septic shock. Thiamine (vitamin B1) is a water-soluble vitamin and a cofactor for pyruvate dehydrogenase enzyme, essential for converting pyruvate to acetyl-coenzyme A for entry into the Krebs cycle. Increased metabolic demand, parenteral or enteral nutrition, diuretics, as well as hemodialysis and hemofiltration can deplete thiamine levels. Thiamine deficiency rates range from 20% to 70% in septic shock patients (5,6). At inadequate levels of thiamine, pyruvate is unable to be converted to acetyl coenzyme A, which results in impaired aerobic respiration and a compensatory shift to the anaerobic pathway. This, in turn, results in elevated serum lactate levels (7). Some studies show that thiamine deficiency may be associated with increased mortality (6,8-10).
Thiamine supplementation has not been associated with serious adverse effects, even at high doses (11). Up to 500 mg per dose IV may be necessary for patients with septic shock. Anaphylaxis has been reported in rare instances (11). Intravenous (IV) thiamine should be administered over a 15- to 30-minute interval in a mixture of saline solution or dextrose to avoid potential adverse reactions (12). However, some studies suggest thiamine administration with doses up to 200 mg as an IV push with no evidence of anaphylactic reactions (13,14). Thiamine supplementation can be a low-risk and potentially high-reward intervention for some patients with septic shock and increased baseline risk of thiamine deficiency.
Findings on the efficacy of IV thiamine are not consistent. In small, retrospective studies of septic ICU patients, the combination of thiamine (200 mg IV every 12 hours), ascorbic acid (1,500 mg IV every six hours), and hydrocortisone (50 mg IV every six hours) improved organ injury, time to shock reversal, increased lactate clearance and decreased mortality (3,15). APACHE-adjusted ICU mortality was lowest when combination therapy was initiated within six hours of presentation with sepsis and in the subgroup of patients with SOFA scores of greater than 10 or with hypoalbuminemia (albumin below 3 g/dL) (16,17).
A prospective, open-label, randomized study by Wani et al. showed no improvement in 30-day mortality or reduction in hospital length of stay; however, vasopressor use was reduced and lactate clearance was improved (18). In a randomized, placebo-controlled clinical trial of septic patients by Fujii et al., standard care showed no difference in median time alive, time to no vasopressor use or 28-day mortality as compared to the combination therapy (IV high-dose ascorbic acid, IV thiamine, and IV hydrocortisone) (19).
In another randomized, double-blind trial of 88 septic shock patients at increased risk of symptomatic thiamine deficiency (serum lactate above 3 mmol/L after volume resuscitation), thiamine had no effect on the primary outcome of median lactate level at 24 hours. In a pre-defined subgroup of patients with thiamine deficiency (35% of the cohort), IV thiamine reduced lactate levels and improved mortality (6). In a before–after study (n=94) by Marik et al., combination therapy of IV ascorbic acid, IV thiamine, and IV steroid reduced mortality (40.4% and 8.5% in control and treatment groups, respectively, P<0.001) (20). A similar before–after study by Kim et al. found a significant reduction in mortality among patients with severe pneumonia (39% vs. 17% in control and treatment groups, respectively, P=0.005) (21).
As far as the duration of therapy goes, the recommendations vary as well. The latest available data suggests that thiamine therapy can be extended beyond 72 hours. The study by Donnino et al. continued thiamine therapy for septic patients for up to 7 days or until hospital discharge (22). Despite the study not finding a statistically significant decrease in lactate levels, shock reversal, or mortality, thiamine group had decreased requirement for CRRT suggesting its benefit in decreasing the risk of acute kidney injury (AKI) in sepsis.
The current study evaluated the effects of IV thiamine 200 mg every 12 hours in patients with sepsis, and septic shock pre- vs. post-implementation of an evidence-based sepsis thiamine guideline. We present this study in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jeccm-21-74).
Methods
Study design and setting
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by institutional review board of Swedish Hospital Part of NorthShore (No. 2020093006).
Individual consent for this retrospective analysis was waived. For the prospective phase of the study, informed consent was obtained from the patients or from patients’ representatives (a friend, family, healthcare proxy, guardian or surrogate) when he or she lacked the capacity to do so.
The study consisted of retrospective (January through July 2020) and prospective (November 2020 through March 2021) phases. In the pre-intervention phase, critical care patients with sepsis or septic shock diagnosis, who did not receive thiamine, were matched with patients who received average daily thiamine dose of 200 mg for the period of January 1st through July 15th, 2020. Twenty-six patients in the control group were matched with 26 patients who received any dose of IV thiamine, with a total of 52 patients in the retrospective phase, based on receiving standard of care (IV fluids and antibiotics within three hours of diagnosis) and ventilation. An evidence-based, thiamine guideline for sepsis, standardizing the dose, duration, and time of initiation was developed, approved by the Pharmacy & Therapeutics Committee and implemented in November. Per the guideline, the post-intervention phase patients received 200 mg of thiamine intravenously every 12 hours within six hours of sepsis or septic shock diagnosis for a minimum of 72 hours or six doses. The guideline recommends thiamine discontinuation upon sepsis or septic shock resolution and/or discontinuation of vasopressors.
Variables
The primary endpoint was hospital mortality. The secondary endpoints were time to death, time to lactate level less than 2 mmol/L, vasopressor use, ICU length of stay, vasopressor duration, renal replacement therapy (RRT) requirement, PaO2/FiO2 ratio at discharge, as well as SOFA score at 72 hours. The inclusion criteria were: age of 18 years or older, critical care unit (ICU/IMCU) admission with sepsis or septic shock diagnosis. The exclusion criteria were: allergy or anaphylactic reaction to thiamine, clinical indication for thiamine (e.g., alcoholism, Wernicke’s encephalopathy), and pregnancy.
In the prospective phase, the same primary and secondary endpoints were then compared between the post-intervention (thiamine dose of 200 mg every 12 hours) and pre-intervention (thiamine at any dose and frequency) thiamine patients, as well as between post-intervention thiamine and control patients.
Statistical methods
Statistical tests for data analysis included chi-square and Fisher’s Exact (when 50% of the values had expected counts less than five for categorical data. Continuous data were analyzed using Wilcoxon Rank Sum test and presented using median and interquartile range. Alpha level for statistical significance was set at 0.05 or less. Time to event analysis was presented as Kaplan-Meier survival curves for time to hospital mortality and time to discharge from critical care unit.
Results
Retrospective phase
In the retrospective phase of the study, out of 345 patients screened 52 patients meeting the eligibility criteria were random selected. For the retrospective pre-intervention phase, baseline characteristics were similar between the thiamine (n=26) and control groups (n=26), except for gender, vasopressor use, AKI at baseline and antibiotic initiation within three hours of sepsis diagnosis (Table 1). Another difference in baseline characteristics between the groups was steroid use: 50% in the control group received steroids vs. about 70% in the thiamine group.
Table 1
| Variable | Control group (n=26) | Pre-intervention thiamine group (n=26) | P value |
|---|---|---|---|
| Age, mean ± SD, years | 65.7±3.3 | 62.6±12.9 | 0.386 |
| Weight, mean ± SD, kg | 84.7±31.5 | 80.6±18.9 | 0.400 |
| Sex, male, n (%) | 22 (84.6) | 16 (61.5) | 0.007 |
| Admission diagnosis, n (%) | |||
| Community acquired pneumonia | 8 (30.8) | 6 (23.1) | 0.395 |
| Pneumonia due to COVID | 9 (34.6) | 11 (42.3) | 0.410 |
| Surgical site infection | 0 (0) | 1 (3.85) | 0.845 |
| UTI/pyelonephritis | 4 (15.4) | 3 (11.5) | 0.587 |
| GI infection | 2 (7.7) | 2 (7.7) | 1.0 |
| HCAP | 0 (0) | 1 (3.8) | 0.845 |
| Respiratory distress | 0 (0) | 2 (7.7) | 0.695 |
| SSTI | 3 (11.5) | 1 (3.8) | 0.220 |
| Unknown | 0 (0) | 1 (3.8) | 0.845 |
| Comorbidities, n (%) | |||
| CAD/MI | 6 (23.1) | 8 (30.8) | 0.3352 |
| Hypertension | 14 (65.4) | 15(57.7) | 0.410 |
| Hyperlipidemia | 7 (26.9) | 11 (42.3) | 0.077 |
| Diabetes | 9 (34.6) | 10 (38.5) | 0.680 |
| Heart failure | 2 (7.7) | 3 (11.5) | 0.462 |
| CVA | 3 (11.5) | 1 (3.85) | 0.220 |
| COPD | 3 (11.5) | 2 (7.7) | 0.539 |
| CKD | 2 (7.7) | 2 (7.7) | 1.0 |
| PVD | 0 (0) | 1 (3.85) | 0.845 |
| Immunocompromised | 5 (19.2) | 4 (15.4) | 0.619 |
| Cirrhosis | 0 (0) | 1 (3.85) | 0.845 |
| Opioid use | 3 (11.5) | 1 (3.85) | 0.220 |
| None/unknown | 3 (11.5) | 4 (15.4) | 0.539 |
| Mechanical ventilation | 18 (69.2) | 21 (80.8) | 0.202 |
| Vasopressors | 14 (53.8) | 24 (92.3) | 0.004 |
| Positive blood cultures | 17 (65.4) | 16 (61.5) | 0.680 |
| Acute kidney injury | 11 (42.3) | 22 (84.6) | 0.005 |
| Lab values | |||
| Lactate, median (IQR), mmol/L | 2.7 (1.3–4.2) | 2.2 (1.5–5.7) | 0.543 |
| Procalcitonin, median (IQR), mcg/mL | 0.51 (0.19–1.94) | 0.81 (0.19–5.00) | 0.488 |
| PaO2/FiO2 ratio, mean ± SD, mmHg | 212.7778±123.5588 | 195.3444±142.7044 | 0.445 |
| Treatment timing and duration | |||
| Fluids within 3 hours of sepsis diagnosis, n (%) | 21 (80.8) | 18 (69.2) | 0.135 |
| Antibiotics within 3 hours of sepsis diagnosis, n (%) | 24 (92.3) | 15 (57.7) | <0.0001 |
| Timing of thiamine initiation, mean ± SD, h | – | 155.2±183 | – |
| Number of thiamine doses, mean ± SD | – | 8.8±5.7 | – |
| Thiamine dose, mean ± SD, mg | – | 220±110 | – |
| Receipt of steroid, n (%) | 13 (50.0) | 18 (69.2) | 0.049 |
| Daily steroid dose (hydrocortisone equivalent), median (IQR), mg | 200 [200–530] | 200 [100–300] | 0.330 |
| Duration of steroid therapy, median (IQR), h | 96 [38–240] | 72 [24–144] | 0.290 |
| COVID presence at diagnosis, n (%) | 11 (42.3) | 12 (46.2) | 0.691 |
UTI, urinary tract infection; SSTI, skin and soft tissue infections; CAD/MI, coronary artery disease/myocardial infarction; CVA, cerebrovascular accident; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; PVD, peripheral vascular disease.
There was no statistically significant difference between the two groups with respect to the primary outcome. There was no difference between groups with respect to secondary outcomes, with the exception of RRT requirements: more patients in control group required RRT as compared to the treatment group (65.4% vs. 42.3%, P=0.013) (Table 2).
Table 2
| Primary outcome | Control group (n=26) | Pre-intervention thiamine group (n=26) | P value |
|---|---|---|---|
| Hospital mortality, n (%) | 9 (34.6) | 10 (38.5) | 0.680 |
| Secondary outcomes | |||
| Time to death, median (IQR), days | 9 [8–21] | 7.5 [3–12] | 0.93 |
| Time to lactate levels <2 mmol/L, median (IQR), hours | 21 [10–24] | 37.5 [24–48] | 0.06 |
| ICU/IMCU length of stay, median (IQR), days | 9 (4–17.3) | 11.5 (6.8–25.3) | 0.479 |
| Vasopressor duration, median (IQR), h | 48 (23.5–117.8) | 51.5 [24–151] | 0.75 |
| RRT required, n (%) | 17 (65.4) | 11 (42.3) | 0.013 |
| PaO2/FiO2 ratio, median (IQR), mmHg | 244 [127–305] | 197 [107–327] | 0.59 |
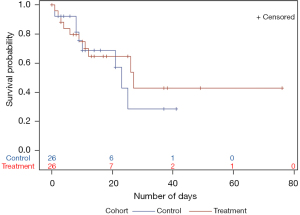
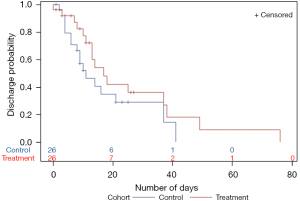
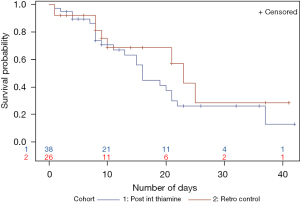
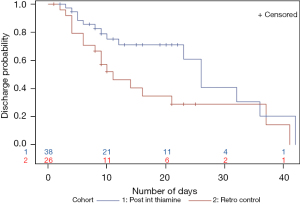
Kaplan-Meier survival analysis for time to death showed that the thiamine group had a higher survival probability. Time to death was longer in the thiamine group, however, the difference was not statistically significant (Figure 1). Kaplan-Meier analysis for time to ICU/IMCU discharge showed that the probability of being discharged from ICU/IMCU was higher in thiamine group compared to the control group with no statistically significant difference (Figure 2). Time to discharge was also longer in thiamine group.
Prospective phase
Pre-intervention thiamine group vs. post-intervention thiamine group
Following thiamine guideline implementation and completion of the prospective post-intervention phase, out of 66 patients, 38 met the eligibility criteria with guideline-directed thiamine use. The primary and secondary outcomes were compared between the pre-intervention thiamine and post-intervention thiamine groups. Baseline characteristics were similar between the two groups, except the timing of antibiotic initiation: less patients in the retrospective thiamine group received antibiotics within 3 hours of sepsis or septic shock diagnosis compared to the post-intervention thiamine group (57.7% vs. 92.1%, P=0.001) (Table 3).
Table 3
| Variable | Pre-intervention thiamine group (n=26) | Post-intervention thiamine group (n=38) | P value |
|---|---|---|---|
| Age, mean ± SD, years | 62.6±12.9 | 67.3±12.9 | 0.11 |
| Weight, mean ± SD, kg | 80.6±18.9 | 81.1±23.9 | 0.85 |
| Sex, male, n (%) | 16 (61.5) | 27 (71.1) | 0.63 |
| Admission diagnosis, n (%) | |||
| Septic shock due to pneumonia | 17 (65.4) | 17 (44.7) | 0.05 |
| Septic shock due to UTI | 3 (11.5) | 3 (3.79) | 0.68 |
| GI infection | 2 (7.7) | 1 (2.6) | 0.99 |
| SSTI | 1 (3.8) | 1 (2.6) | 0.99 |
| Comorbidities, n (%) | |||
| CAD/MI | 8 (30.8) | 5 (13.2) | 0.09 |
| Hypertension | 15 (57.7) | 24 (63.2) | 0.66 |
| Hyperlipidemia | 11 (42.3) | 18 (47.4) | 0.69 |
| Diabetes | 10 (38.5) | 15 (39.5) | 0.94 |
| Heart failure | 3 (11.5) | 6 (15.8) | 0.73 |
| COPD | 2 (7.7) | 3 (7.9) | 0.98 |
| CKD | 2 (7.7) | 6 (15.8) | 0.46 |
| Immunocompromised | 4 (15.4) | 5 (13.2) | 0.80 |
| None/unknown | 4 (15.4) | 4 (10.5) | 0.71 |
| Other, n (%) | |||
| Mechanical ventilation | 21 (80.8) | 34 (89.5) | 0.29 |
| Vasopressors | 24 (92.3) | 38 (100) | 0.16 |
| Positive blood cultures | 16 (61.5) | 20 (52.6) | 0.48 |
| Acute kidney injury | 22 (84.6) | 26 (68.4) | 0.27 |
| Lab values | |||
| Lactate, median (IQR), mmol/L | 2.2 (1.5–5.7) | 3.7 (2.2–8.3) | 0.06 |
| PaO2/FiO2 ratio, mean ± SD, mmHg | 195.3±142.7 | 134.5±80.3 | 0.20 |
| SOFA score on day 1, median (IQR) | 7 (4.7–9.5) | 9 (7.8–9) | 0.22 |
| Treatment timing and duration | |||
| Fluids within 3 hours of sepsis diagnosis, n (%) | 18 (69.2) | 29 (76.3) | 0.53 |
| Antibiotics within 3 hours of sepsis diagnosis, n (%) | 15 (57.7) | 35 (92.1) | 0.001 |
| Number of thiamine doses, mean ± SD | 8.8±5.7 | 10±6.9 | 0.31 |
| Daily thiamine dose, mean ± SD, mg | 220±110 | 400±0 | – |
| Received steroids, n (%) | 18 (69.2) | 32 (84.2) | 0.15 |
| Daily steroid dose (hydrocortisone equivalent), median (IQR), mg | 200 [100–300] | 200 [200–200] | 0.80 |
| Duration of steroid therapy, median (IQR), h | 72 [24–144] | 99 [72–192] | 0.10 |
| COVID presence at diagnosis, n (%) | 12 (46.2) | 18 (71.1) | 0.92 |
UTI, urinary tract infection; SSTI, skin and soft tissue infections; CAD/MI, coronary artery disease/myocardial infarction; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease.
In the prospective phase, the post-intervention thiamine group with standardized thiamine dosing did not reveal any mortality benefit (Table 4). In addition, no difference in secondary outcomes between the pre-intervention and post-intervention thiamine groups were observed.
Table 4
| Primary outcome | Pre-intervention thiamine group (n=26) | Post-intervention thiamine group (n=38) | P value |
|---|---|---|---|
| Hospital mortality, n (%) | 10 (38.5) | 23 (60.5) | 0.08 |
| Secondary outcomes | |||
| Time to death, median (IQR), days | 7.5 [3–12] | 11.5 [7–21] | 0.26 |
| Time to lactate levels <2 mmol/L, median (IQR), hours | 37.5 [24–48] | 28 [18–86] | 0.47 |
| ICU/IMCU length of stay, median (IQR), days | 11.5 (6.8–25.3) | 13 [8–19] | 0.86 |
| Vasopressor duration, median (IQR), h | 51.5 [24–151] | 79.8 [34–203] | 0.29 |
| RRT required, n (%) | 11 (42.3) | 14 (36.8) | 0.66 |
| PaO2/FiO2 ratio, median (IQR), mmHg | 197 [107–327] | 178 [93–250] | 0.77 |
| SOFA score at 72 hours | 8 (6.5–12) | 8 (4.8–12) | 0.90 |
ICU, intensive care unit; IMCU, intermediate care unit.
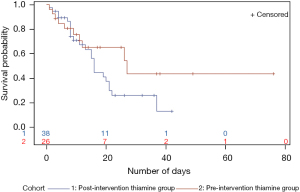
Time to hospital death Kaplan-Meier analysis did not show any benefit in survival probability for patients treated with guideline-directed thiamine therapy: time to death was shorter in this group (Figure 3).
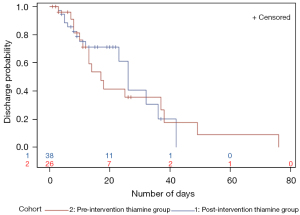
Same pattern was observed for time to critical care unit discharge with the post-intervention thiamine group not showing any benefit in discharge probability or time to ICU/IMCU discharge (Figure 4). When looking at the post-intervention thiamine group curve (blue line), it is worth mentioning some observed benefit around days 10 to 30. However, the curve for the pre-intervention thiamine group (red line) is longer, mostly because the patients who survived stayed longer in the critical care units.
Control vs. post-intervention thiamine group
The control (n=26) and post-intervention thiamine groups (n=38) had some differences in baseline characteristics. It is worth noting that at baseline, all patients in post-intervention thiamine group required vasopressors at baseline, had higher lactate levels, higher SOFA scores, and lower PaO2/FiO2 ratio (Table 5). When comparing the two groups, the primary outcome of hospital mortality showed difference in favor of the control group (34.6% vs. 60.5% control vs. post-intervention thiamine groups, respectively; P=0.04) (Table 6).
Table 5
| Variable | Control (n=26) | Post-intervention thiamine group (n=38) | P value |
|---|---|---|---|
| Age, mean ± SD, years | 65.7±16.8 | 67.3±12.9 | 0.58 |
| Weight, mean ± SD, kg | 84.7±31.5 | 81.1+ 23.9 | 0.87 |
| Sex, male, n (%) | 22 (84.6) | 27 (71.1) | 0.21 |
| Admission diagnosis, n (%) | |||
| Septic shock due to pneumonia | 9 (34.6) | 17 (44.7) | 0.42 |
| Septic shock due to UTI | 4 (15.4) | 3 (7.9) | 0.43 |
| GI infection | 2 (7.7) | 1 (2.6) | 0.56 |
| SSTI | 3 (11.5) | 1 (2.6) | 0.30 |
| Comorbidities, n (%) | |||
| CAD/MI | 6 (23.1) | 5 (13.2) | 0.30 |
| Hypertension | 14 (53.8) | 24 (63.2) | 0.46 |
| Hyperlipidemia | 7 (26.9) | 18 (47.4) | 0.10 |
| Diabetes | 9 (34.6) | 15 (39.5) | 0.69 |
| Heart failure | 2 (7.7) | 6 (15.8) | 0.34 |
| COPD | 3 (11.5) | 3 (7.9) | 0.62 |
| CKD | 2 (7.7) | 6 (15.8) | 0.34 |
| Immunocompromised | 5 (19.2) | 5 (13.2) | 0.51 |
| None/unknown | 3 (11.5) | 4 (10.5) | 0.90 |
| Other, n (%) | |||
| Mechanical ventilation | 18 (69.2) | 34 (89.5) | 0.05 |
| Vasopressors | 16 (61.5) | 38 (100) | <.0001 |
| Positive blood cultures | 17 (65.4) | 20 (52.6) | 0.31 |
| Acute kidney injury | 11 (42.3) | 26 (68.4) | 0.04 |
| Lab values | |||
| Lactate, median (IQR), mmol/L | 2.7 (1.3–4.1) | 3.7 (2.2–8.2) | 0.01 |
| PaO2/FiO2 ratio, mean ± SD, mmHg | 212.3±123.6 | 134.5±80.3 | 0.02 |
| SOFA score on day 1, median (IQR) | 6.5 [4–8] | 9 [7–10] | 0.045 |
| Treatment timing and duration | |||
| Fluids within 3 hours of sepsis diagnosis, n (%) | 21 (80.8) | 29 (76.3) | 0.67 |
| Antibiotics within 3 hours of sepsis diagnosis, n (%) | 24 (92.3) | 35 (92.1) | 0.98 |
| Number of thiamine doses, mean ± SD | – | 10±6.9 | – |
| Daily thiamine dose, mean ± SD, mg | – | 400±0 | – |
| Received steroids, n (%) | 13 (50.0) | 32 (84.2) | 0.003 |
| Daily steroid dose (hydrocortisone equivalent), median (IQR), mg | 200 [200–530] | 200 [200–200] | 0.06 |
| Duration of steroid therapy, median (IQR), h | 96 [38–240] | 99 [72–192] | 0.53 |
| COVID presence at diagnosis, n (%) | 11 (42.3) | 18 (71.1) | 0.69 |
UTI, urinary tract infection; SSTI, skin and soft tissue infections; GI, gastrointestinal; CAD/MI, coronary artery disease/myocardial infarction; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease.
Table 6
| Primary outcomes | Control (n=26) | Post-intervention thiamine group (n=38) | P value |
|---|---|---|---|
| Hospital mortality, n (%) | 9 (34.6) | 23 (60.5) | 0.04 |
| Secondary outcomes | |||
| Time to death, median (IQR), days | 9 [8–21] | 13 [8–19] | 0.83 |
| Time to lactate levels <2 mmol/L, median (IQR), hours | 18 [10–24] | 28 [18–84] | 0.08 |
| ICU/IMCU length of stay, median (IQR), days | 9 [4–16] | 11.5 [7–21] | 0.28 |
| Vasopressor duration, median (IQR), h | 47.5 (23.0–94.5) | 79.8 (37.0–194.5) | 0.18 |
| RRT required, n (%) | 17 (65.4) | 14 (36.8) | 0.02 |
| PaO2/FiO2 ratio, median (IQR), mmHg | 244 [127–305] | 178.6 (95.9–334.0) | 0.41 |
| SOFA score at 72 hours, median (IQR) | 7 [6–9] | 8 [7–10] | 0.41 |
ICU, intensive care unit; IMCU, intermediate care unit.
For the survival analysis, survival probability was similar during the first 10 days of the hospital stay. After the 10-day mark, the post-intervention thiamine group did worse. The survival probability for control vs. post-intervention thiamine groups were similar around day 25. However, around day 35 the post-intervention thiamine patients had a lower survival probability (Figure 5). Time to critical care unit discharge was not different between the two groups (Figure 6).
Discussion
More patients in the control group were initiated on antibiotics in a timely manner compared to the thiamine group during the retrospective phase. While this is a confounding factor, it is unknown to what extent this contributes to the frequency of the primary outcome in each group.
Differences in some baseline characteristics in the pre-intervention group along with the small sample size limit the generalizability of the study results. In the retrospective phase, time to discharge was also longer in thiamine group, which can be explained by the fact that patients who survived were more likely to stay in ICU or IMCU before being discharged or downgraded.
In the post-intervention phase, when comparing the pre-intervention thiamine group with the post-intervention thiamine group, there was no difference in baseline characteristics except for more timely antibiotic initiation in the post-intervention thiamine group. When comparing the retrospective control group with post-intervention thiamine group, the groups were significantly different in terms of some key baseline characteristics such as the number of patients on vasopressors, baseline lactate levels, SOFA score, as well as PaO2/FiO2 ratios. As the post-intervention thiamine group had sicker patients with higher lactate levels, higher SOFA scores, and lower PaO2/FiO2 ratio, this can help explain the lack of mortality benefit in the intervention group.
Conclusions
In the retrospective or pre-intervention phase of the current study, thiamine groups did not show benefit compared with the control group with respect to primary and most of the secondary outcomes, with the exception of RRT requirement. This can be a potential benefit of thiamine therapy, suggesting renally protective effects in critically ill patients. However, RRT requirement was a secondary outcome of the retrospective phase of the study and the latter finding is only exploratory in nature. This finding of the present study corresponds to findings from a randomized, double-blind trial, where thiamine reduced the need for RRT (23).
As for the prospective or post-intervention phase, the study did not find statistically significant differences between standardized and unspecified thiamine dosing strategies. While this study was unable to demonstrate a mortality impact, given the risk vs. benefit profile, thiamine may be considered with current study limitations. Giving some thiamine may have masked the effect of guideline-based thiamine, decreasing the magnitude of the difference between no thiamine and thiamine at evidence-based doses.
Study limitations, such as small sample size and different baseline characteristics for some variables, limit applicability of study results. However, of note, the post-intervention thiamine group had sicker patients, which could be a contributing factor to more timely antibiotic initiation. Since this is one of the most important driving factors in reducing mortality, that benefit could have outshined the benefits of a standardized thiamine dosing approach. It is an important confounder that could not be adequately controlled for in this study. Even a marginal benefit of thiamine in this patient population can make a difference in mortality with a properly conducted large-scale study.
Thiamine with or without IV steroids and vitamin C may be a promising therapy for sepsis or septic shock, however, currently lacks robust evidence to support its use. The rationale behind using thiamine in this patient population is supported by its effect on the pentose phosphate pathway, facilitating carbohydrate metabolism and shifting anaerobic cellular metabolism to aerobic (5-7).
Future studies should further investigate thiamine effects on hospital mortality in septic patients when combined with other therapies, such as IV steroids and vitamin C. Another potential area of investigation for thiamine benefits can be septic patients with renal dysfunction.
Acknowledgments
The authors thank Andrew Katsoulos for providing patient reports in the retrospective phase of the study, Lavisha Singh for helping with data analysis and Alicia Juska, PharmD, BCPS for her guidance throughout the study period.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jeccm-21-74
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jeccm-21-74
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jeccm-21-74). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Swedish Hospital Part of NorthShore (No. 2020093006) and individual consent for this retrospective analysis was waived. For the prospective phase of the study, informed consent was obtained from the patients or from patients’ representatives (a friend, family, healthcare proxy, guardian or surrogate) when he or she lacked the capacity to do so.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rhee C, Dantes R, Epstein L, et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. JAMA 2017;318:1241-9. [Crossref] [PubMed]
- Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med 2004;351:159-69. [Crossref] [PubMed]
- Moskowitz A, Andersen LW, Huang DT, et al. Ascorbic acid, corticosteroids, and thiamine in sepsis: a review of the biologic rationale and the present state of clinical evaluation. Crit Care 2018;22:283. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 2017;45:486-552. [Crossref] [PubMed]
- Donnino MW, Carney E, Cocchi MN, et al. Thiamine deficiency in critically ill patients with sepsis. J Crit Care 2010;25:576-81. [Crossref] [PubMed]
- Donnino MW, Andersen LW, Chase M, et al. Randomized, Double-Blind, Placebo-Controlled Trial of Thiamine as a Metabolic Resuscitator in Septic Shock: A Pilot Study. Crit Care Med 2016;44:360-7. [Crossref] [PubMed]
- Andersen LW, Holmberg MJ, Berg KM, et al. Thiamine as an adjunctive therapy in cardiac surgery: a randomized, double-blind, placebo-controlled, phase II trial. Crit Care 2016;20:92. [Crossref] [PubMed]
- Mallat J, Lemyze M, Thevenin D. Do not forget to give thiamine to your septic shock patient! J Thorac Dis 2016;8:1062-6. [Crossref] [PubMed]
- Cruickshank AM, Telfer AB, Shenkin A. Thiamine deficiency in the critically ill. Intensive Care Med 1988;14:384-7. [Crossref] [PubMed]
- Corcoran TB, O'Neill MA, Webb SA, et al. Prevalence of vitamin deficiencies on admission: relationship to hospital mortality in critically ill patients. Anaesth Intensive Care 2009;37:254-60. [Crossref] [PubMed]
- Donnino MW, Vega J, Miller J, et al. Myths and misconceptions of Wernicke's encephalopathy: what every emergency physician should know. Ann Emerg Med 2007;50:715-21. [Crossref] [PubMed]
- Galvin R, Bråthen G, Ivashynka A, et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol 2010;17:1408-18. [Crossref] [PubMed]
- Tjugum SL, Hedrick TL, Jean SJ, et al. Evaluation of the Safety of Intravenous Thiamine Administration in a Large Academic Medical Center. J Pharm Pract 2021;34:397-402. [Crossref] [PubMed]
- McLaughlin K, Joyal K, Lee S, et al. Safety of intravenous push thiamine administration at a tertiary academic medical center. J Am Pharm Assoc (2003) 2020;60:598-601. [PubMed]
- Woolum JA, Abner EL, Kelly A, et al. Effect of Thiamine Administration on Lactate Clearance and Mortality in Patients With Septic Shock. Crit Care Med 2018;46:1747-52. [Crossref] [PubMed]
- Long MT, Kory P, Marik P, Vitamin C. Hydrocortisone, and Thiamine for Septic Shock. JAMA 2020;323:2203-4. [Crossref] [PubMed]
- Hwang SY, Park JE, Jo IJ, et al. Combination therapy of vitamin C and thiamine for septic shock in a multicentre, double-blind, randomized, controlled study (ATESS): study protocol for a randomized controlled trial. Trials 2019;20:420. [Crossref] [PubMed]
- Wani SJ, Mufti SA, Jan RA, et al. Combination of vitamin C, thiamine and hydrocortisone added to standard treatment in the management of sepsis: results from an open label randomised controlled clinical trial and a review of the literature. Infect Dis (Lond) 2020;52:271-8. [Crossref] [PubMed]
- Fujii T, Luethi N, Young PJ, et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients With Septic Shock: The VITAMINS Randomized Clinical Trial. JAMA 2020;323:423-31. [Crossref] [PubMed]
- Marik PE, Khangoora V, Rivera R, et al. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017;151:1229-38. [Crossref] [PubMed]
- Kim WY, Jo EJ, Eom JS, et al. Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: Propensity score-based analysis of a before-after cohort study. J Crit Care 2018;47:211-8. [Crossref] [PubMed]
- Donnino M, Andersen L, Chase M, et al. Thiamine as a metabolic resuscitator in septic shock: a randomized, double-blind, placebo-controlled, pilot trial. Crit Care 2015;19:392. [Crossref]
- Moskowitz A, Andersen LW, Cocchi MN, et al. Thiamine as a Renal Protective Agent in Septic Shock. A Secondary Analysis of a Randomized, Double-Blind, Placebo-controlled Trial. Ann Am Thorac Soc 2017;14:737-41. [Crossref] [PubMed]
Cite this article as: Sahakian Y, Dyakov D. The effects of thiamine on patients with sepsis and septic shock. J Emerg Crit Care Med 2022;6:1.


