False blood leak alarm from dialysate discoloration following vitamin B12 infusion: case report
Introduction
Hemodialysis blood leaks may result in significant blood loss and hemorrhagic shock in patients requiring hemodialysis. To avoid such complications hemodialysis machines are fitted with sensors that stop the dialysis machine when blood is detected in the dialysate. Vitamin B12 is readily removed by hemodialysis and may appear in the dialysate, where it produces a red discoloration. The change in dialysate color can activate the blood leak alarm in dialysis machines relying on the absorption of transmitted light in pure transmission mode rather than scattering and adsorption to detect cellular components (i.e., blood cells) in the dialysate. Changing to a hemodialysis machine that utilizes a single optical emitter to detect blood leaks can overcome this situation, as single optical emitters do not activate in the presence of chromophores. Early recognition of this potential complication is essential in managing renal replacement therapy in critically ill patients, as only certain dialysis machines can be utilized. We present the following case in accordance with the CARE reporting checklist (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-22-1/rc).
Case presentation
A 55-year-old Hispanic male with renal failure ascribed to diabetic kidney disease presented to the emergency room with chest pain radiating to the left flank and shortness of breath associated with orthopnea over the past eight hours.
His past medical history was notable for type II diabetes mellitus complicated by renal failure, retinopathy, and peripheral arterial disease resulting in two prior toe amputations, hypertension, benign prostatic hyperplasia, anemia, and secondary hyperparathyroidism. In the emergency room, he was diaphoretic with a temperature of 36.5 ℃, blood pressure of 107/74 mmHg, pulse of 91 beats per minute, and respiratory rate of 28 per minute with SpO2 of 86% on room air. He was placed on 6-L nasal cannula and his oxygen saturation improved to 99%. A chest X-ray revealed left lower lobe ground glass opacities, and a computed tomography angiography was negative for pulmonary embolism. A rapid COVID-19 nasopharyngeal PCR was negative. A 12-lead electrocardiogram (EKG) showed ST depressions in V4-6, and his initial troponin was 1.1 ng/mL. Repeat troponin testing showed an increase of 6.6 ng/mL, and an echocardiogram revealed new akinesis of the apical myocardium and hypokinesis of the entire anterior, mid anteroseptal, basal-mid anterolateral, and apical lateral myocardium and an ejection fraction (EF) of 35%.
He underwent emergent left heart cardiac catheterization which demonstrated 50% proximal stenosis of the left anterior descending artery (LAD), 80% ostial lesion, 70% proximal and mid-right coronary artery lesion, and diffusely calcific left circumflex artery. Clopidogrel and aspirin were administered, he was started on heparin and transferred for a higher level of care. He underwent hemodialysis without complications, his respiratory status improved, and he was taken off supplemental oxygen. Cardiothoracic surgery was consulted and it was recommended he undergo a coronary artery bypass graft surgery (CABG). Due to operating room scheduling difficulties, he was discharged on hospital day #9 with plans for outpatient surgery in 1 week. He was admitted for his scheduled surgery without symptoms and underwent 5 vessel CABG. The initial portion of the surgery was uncomplicated. However, upon removal of bypass, he developed intra-operative depressed cardiac function with right ventricular failure. An intra-aortic balloon pump was placed without improved cardiac function, and the patient was subsequently placed on extracorporeal membrane oxygenation (ECMO). Post-operatively he was transferred to the intensive care unit (ICU) with hypotension. He required epinephrine, vasopressin, phenylephrine, and norepinephrine drips to maintain mean arterial pressure (MAP) >60 mmHg. An echocardiogram revealed a post-procedure EF of 1–10%. Five grams of vitamin B12 were administered for ongoing cardiogenic shock.
Upon transfer to the ICU, laboratory evaluation revealed serum potassium of 6.3 mmol/dL, and intermittent hemodialysis (IHD) was ordered through his left forearm arteriovenous fistula using a Fresenius 2008K machine. Shortly after starting IHD, the blood leak alarm triggered and the dialysis machine stopped. Examination revealed an intact dialyzer and normal arterial, venous, and transmembrane pressures prior to leak detection. He underwent a trial of hemodialysis with a different Fresenius 2008K dialysis machine; however, the blood leak alarm was triggered and dialysis was halted.
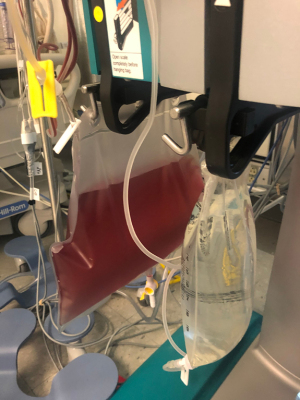
Given the recent administration of high doses of vitamin B12 a false blood leak alarm was suspected and it was decided to use another type of dialysis machine (PRISMA) which utilizes a single optical emitter sensor and does not activate in the presence of visible spectrum chromophores in the dialysate. A right internal jugular dialysis catheter was placed and continuous renal replacement therapy (CRRT) was initiated. The dialysate collection showed a red effluent which did not show any red blood cells on microscopic examination, confirming a false blood leak (Figure 1). Over the next 24 hours, the patient’s dialysate progressively cleared as the vitamin B12 was removed by dialysis.
The patient’s course continued to deteriorate. He returned to the operating room on postoperative day #1 for clot removal from the right ventricle, and on postoperative day #4, he required a right femoral artery repair and ECMO decannulation. Following his return to the ICU, he expired.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written consent was not obtained as the patient expired and unable to reach family member.
Discussion
Hemodialysis removes uremic solutes by exposing blood to a chemically different solution called dialysate. The two fluids are physically separated by a semi-permeable membrane with diffusion of solutes occurring by a concentration gradient. Damage to the dialyzer membrane can result in mixing of blood and non-sterile dialysate, termed a “blood leak”, and results in a “port wine” discoloration of the dialysate. Blood leaks can result in excessive blood loss from the patient, resulting in hemorrhagic shock (1). The infusion of non-sterile dialysate into the patient may also result in significant electrolyte imbalances, and may result in life-threatening hemolysis if the osmolarity of the dialysate is significantly different compared to the treated patients’ plasma (2).
To avoid the potential complications of a blood leak, hemodialysis machines are equipped with sensors in the dialysate outflow line specifically designed to detect blood. The blood leak system function by emitting light through passing dialysate, either visible spectrum light, usually red-green, or non-visible infrared wavelength, and measuring changes in light absorption. When preprogrammed, alterations in light absorption trigger the blood leak alarm, which stops the hemodialysis pump, effectively stopping hemodialysis to avoid further blood loss. Non-blood contaminants may result in false blood alarms. The presence of air leaks, dialyzed components in patients with hemolysis, and dialyzable chromophores can result in alterations in light emission and activation of the blood leak sensor (3).
Vitamin B12 is an essential water-soluble vitamin involved in many cellular pathways. Administration of large doses of vitamin B12 can result in an increase in renal removal and increase in urinary concentrations resulting in a visible change in urine color from yellow to red. Vitamin B12 has a molecular weight of 1,355 Daltons and is readily dialyzable due to its small size and water solubility. Supplementation, in combination with other readily dialyzable vitamins, is commonly prescribed for patients on chronic hemodialysis. High doses of vitamin B12 have been used as rescue therapy for refractory shock, as seen in the case presented, and in the treatment of cyanide poisoning for patients in the acute care setting (4). The proposed rationale behind its utilization in persistent vasoplegic syndrome post cardiac surgery is related to the nitric oxide (NO) synthase inhibition, scavenging NO, modifying hydrogen sulfide on NO, and vascular smooth muscle activity (5).
In the case presented, the dialysate turned a red color but no blood cells could be visualized in the dialysate, indicating a false blood leak. PrismaFlex, NxStage and Gambro Phoenix X36 dialysis machines utilize a single optical emitter using infrared wavelengths and are not influenced by visible spectrum dialyzable chromophores, which allowed the patient in our case to undergo CRRT (6). Others have reported false blood leak alarms on IHD machines in patients undergoing hemodialysis shortly following administration of vitamin B12 due to the production of red dialysate with successful hemodialysis after converting to CRRT machines (7-11).
Blood leaks may result in catastrophic complications in patients requiring hemodialysis. However, false alarms may occur following the administration of high doses of IV vitamin B12. Early recognition of this potential complication is essential in managing hemodialysis in critically ill patients as only certain dialysis machines can be utilized in this setting to provide renal replacement therapy. The clinical care team may have a heightened level of suspicion of a vitamin B12 false leak by observing a red discoloration of the dialysate without red cells.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-22-1/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-22-1/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written consent was not obtained as the patient expired and unable to reach family member.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Seabra VF, Jaber BL. Acute Complications During Hemodialysis. In: Floege J, Johnson RJ, Feehally J. editors. Comprehensive Clinical Nephrology. 4th edition. Elsevier, 2010:1069-80.
- Lindley E, Finney D, Jones P, et al. Unexpected triggering of the dialysate blood leak detector by haemolysis. Acta Clin Belg 2015;70:226-9. [Crossref] [PubMed]
- Yeun JY, Depner TA, Ananthakrishnan S. Principles of Hemodialysis. In: Himmelfarb J, Ikizler TA. Chronic Kidney Disease, Dialysis, and Transplantation: A Companion to Brenner and Rector's The Kidney. 4th edition. Elsevier, 2019:339-60.
- Shepherd G, Velez LI. Role of hydroxocobalamin in acute cyanide poisoning. Ann Pharmacother 2008;42:661-9. [Crossref] [PubMed]
- Busse LW, Barker N, Petersen C. Vasoplegic syndrome following cardiothoracic surgery-review of pathophysiology and update of treatment options. Crit Care 2020;24:36. [Crossref] [PubMed]
- Abdelmalek J, Thornton S, Nizar J, et al. Successful use of continuous renal replacement therapy after hydroxocobalamin administration. Dial Transplant 2011;40:415-7. [Crossref]
- Datar P, Sidhu JS, Virk J, et al. A Case of Hydroxocobalamin-Induced False Blood Leak Alarm on Dialysis Machine. J Investig Med High Impact Case Rep 2019;7:2324709619883466. [Crossref] [PubMed]
- Sutter ME, Clarke ME, Cobb J, et al. Blood leak alarm interference by hydoxocobalamin is hemodialysis machine dependent. Clin Toxicol (Phila) 2012;50:892-5. [Crossref] [PubMed]
- Lim K, Heher E, Steele D, et al. Hemodialysis failure secondary to hydroxocobalamin exposure. Proc (Bayl Univ Med Cent) 2017;30:167-8. [Crossref] [PubMed]
- Sutter M, Tereshchenko N, Rafii R, et al. Hemodialysis complications of hydroxocobalamin: a case report. J Med Toxicol 2010;6:165-7. [Crossref] [PubMed]
- Cheungpasitporn W, Hui J, Kashani KB, et al. High-dose hydroxocobalamin for vasoplegic syndrome causing false blood leak alarm. Clin Kidney J 2017;10:357-62. [Crossref] [PubMed]
Cite this article as: Lawson BO, Fahim P, Shen JI, Lum EL. False blood leak alarm from dialysate discoloration following vitamin B12 infusion: case report. J Emerg Crit Care Med 2022;6:15.