
Rescue veno-arterial extracorporeal membrane oxygenation in venlafaxine overdose: a case report
Introduction
Each year close to one million people die by suicide across the world, with drug ingestion a leading cause (1). The antidepressant venlafaxine (VEN) is a bicyclic phenylethylamine antidepressant that inhibits serotonin, norepinephrine, and dopamine reuptake in the central nervous system (CNS). Morbidity and mortality in VEN overdose is manifested by seizures and cardiovascular toxicity (2). Management of VEN overdose has previously focused on supportive care. A growing body of literature explores the use of rescue extracorporeal membrane oxygenation (ECMO) (3-5), as cardiovascular collapse remains the leading cause of death in acute drug intoxication (6).
We describe a case of massive overdose with sustained-release (SR) VEN leading to cardiogenic collapse requiring ECMO. Our case demonstrates the first reported use of ECMO in the United States to successfully treat refractory cardiogenic shock due to VEN overdose. This case also reports the highest ratio of VEN concentration to primary metabolite O-desmethylvenlafaxine (ODV), and contributes to the growing body of literature investigating the use of ECMO in cardiac poisonings. We present the following case in accordance with the CARE reporting checklist (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-22-2/rc).
Case presentation
A 46-year-old woman (60.3 kg) with a history of depression, alcohol and opioid use disorder presented to the emergency department (ED) via ambulance approximately 2.5 hours after last known well (LKW). Emergency Medical Services (EMS) reported an ingestion of 30 pills of VEN (SR) 75 mg (2.25 g) although total ingestion is unclear. The patient recently filled a prescription for 90 tablets of 75 mg VEN.
In the ED, the patient was somnolent, opening eyes to voice and moving all four extremities. Beyond mild tachycardia (Figure 1), there were no significant anticholinergic exam findings. Initial labs notable for hypokalemia to 2.5 mEq/L, hyperglycemia to 285 mg/dL, anion gap (AG) 18 mEq/L, and ethanol (ETOH) 225 mg/dL. Poison control was consulted on arrival. Approximately 3.5 hours after arrival (5.5 hours after LKW), the patient had a tonic-clonic seizure that was self-limited and resolved in less than 3 minutes before returning to baseline. At 6.5 hours from arrival, the patient seized again and was treated effectively with 2 mg intravenous (IV) lorazepam and loaded with 3 g levetiracetam.
One hour later, the patient had a third seizure treated with 2 mg IV lorazepam. The patient became hypotensive with systolic blood pressure <90 mmHg. Point of care transthoracic echocardiogram (TTE) showed a depressed ejection fraction (EF) with apical hypokinesis concerning for a stress-induced cardiomyopathy. A repeat electrocardiogram (ECG) was performed, notable for a widening QRS and prolonged QTc of 559 milliseconds (ms) (Figure 1). Troponin measurements were <0.01 on two separate occasions spaced 6 hours apart.
Despite ongoing fluid resuscitation totaling 3 liters, the patient continued to be hemodynamically unstable in status epilepticus and was intubated prior to admission to the medical intensive care unit. The patient was started on vasopressors for treatment of presumed cardiogenic shock. Formal TTE at 15 hours after ingestion confirmed a depressed left ventricular ejection fraction (LVEF) of 10% with moderate right ventricular systolic dysfunction. By this time, the patient was supported with dobutamine 5 µg/kg/minute, vasopressin 0.03 unit/minute, epinephrine 0.4 µg/kg/minute and norepinephrine 0.5 µg/kg/minute. The hospital ECMO team was consulted for evaluation of refractory cardiogenic shock.
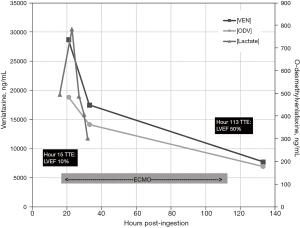
Nineteen hours after ingestion the patient was successfully placed on peripheral veno-arterial (VA) ECMO. Initial serum concentrations of venlafaxine [VEN] and its primary metabolite [ODV] were 28,000 ng/mL and 484 ng/mL respectively, drawn 21 hours after ingestion. Once cannulated, the patient stabilized with improving hemodynamics and a down-trending lactate which peaked at 9.8 mmol/L. On hospital day 5, LVEF improved to 50–55% and ECMO support was discontinued. On hospital day 7, the patient was extubated to room air without complication. The patient was discharged home on hospital day 25 after a full neurological recovery.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
VEN was developed in 1994 as an alternative for patients who did not respond to selective serotonin reuptake inhibitors, with fewer anticholinergic and CNS adverse effects when compared to tricyclic antidepressants. In patients with major depression the recommended doses are 37.5–375 mg/day (7). There are two formulations, immediate-release (IR) and extended-release (ER) or SR. The SR formulation when taken at a dose of 150 mg once daily reaches a peak concentration of 150 ng/mL in 5.5 hours, while its metabolite, ODV, peaks at 260 mg/mL at 9 hours. The IR formulation at 75 mg twice daily has a peak [VEN] and [ODV] at 225 ng/mL at 2 hours and 290 ng/mL at 3 hours respectively (8). It is metabolized in the liver primarily by two CYP450 isoenzymes, 2D6 and 2C19 to its primary active metabolite ODV (9). Both VEN and ODV have a relatively large volume of distribution (7.5±3.7 and 5.7±1.8 L/kg) (8) making them poor candidates for removal via hemodialysis. Furthermore, genetic polymorphisms in CYP2D6 and CYP2C19 have been found to inhibit VEN metabolism and increase risk of toxic adverse effects (9).
More severe consequences of a VEN overdose include seizures and cardiogenic collapse. A retrospective study performed in 2020 examined 953 cases of VEN overdoses in the California Poison Control database and found that seizures occurred in 12.9% of cases (2). Ingestions of greater than 8 g have been shown to be associated with severe cardiotoxicity (9). While our patient reportedly ingested 2.25 g, initial [VEN] suggests a much higher dose (Figure 2). Several case reports have addressed instances of acute systolic heart failure in the setting of VEN overdose, with two main hypotheses as to the etiology. The first theory, which is supported in animal models, hypothesizes an excessive adrenergic stimulation of the heart secondary to increased circulating norepinephrine and catecholamines causing a similar pathology to Takotsubo cardiomyopathy (10). The second theory hypothesizes that myocardial stunning occurs through the inhibition of the inward sodium current, resulting in subsequent inhibition of the cardiac action potential (11).

Our case speculated an ingestion considerably lower than prior ingestions documented in the literature (3,9,12,13) (Figure 2). However, the [VEN] drawn 21 hours post-ingestion suggests either a much higher ingestion dose or may indicate that our patient was a slow metabolizer via CYP450 (Figure 3). At 21 hours after reported ingestion, our patient had a [VEN] =28,700 ng/mL and [ODV] =484 ng/mL measured by Liquid Chromatography-Tandem Mass Spectrometry, a ratio of 60:1 which is considerably higher than previously reported in the literature. Over a 133-hour course of analysis, [VEN] decreased to 7,740 ng/mL and [ODV] to 179 ng/mL. This initial VEN concentration indicates a much higher ingestion when compared to Castanares-Zapatero et al. (12) which reported a [VEN] of 46,094 ng/mL after a 4.2 g overdose on extended release, and Stefani et al. (13) which reported a VEN concentration of 44,197 ng/mL after a 25.2 g overdose on extended release.
VEN overdoses are historically treated with supportive measures. Given the drug’s formulation and toxicokinetics, VEN is not amenable to dialysis (8). Previously, cardiogenic collapse associated with VEN overdose was treated with inotropic agents and vasopressors. Stefani et al. reports the successful use of high-dose insulin euglycemic therapy in the setting of a 25.2 g ingestion to reduce catecholamine exposure (13). Several case reports described the potential use of IV lipid emulsion therapy as an additional scavenging agent in overdose (3,14,15).
Over the last two decades, hospitals have increasingly utilized VA-ECMO for refractory cardiogenic shock in the setting of drug overdose. A review from 2013 highlighted 30 cases of cardiogenic shock or cardiac arrest due to a variety of ingested cardiotoxins, treated with VA-ECMO (6). Approximately 20 survived without serious sequelae.
Fifteen of these patients were eventually decannulated from ECMO, with a majority of them having a favorable cardiovascular and neurological status. The mean ECMO duration was 4.5±2.4 days. More recently, ECMO has shown utility in the specific setting of VEN overdose. Three recent case reports have detailed the successful treatment of VEN overdose with ECMO (3-5).
Conclusions
Suicide by drug ingestion is a leading cause of death across the globe. Cardiovascular collapse remains the primary cause of mortality in these presentations. Our case report demonstrates the first reported use of ECMO in the United States to successfully treat refractory cardiogenic shock secondary to VEN overdose. Our report also describes the highest [VEN]:[ODV] ratio and contributes to the growing body of literature to support the use of ECMO in cardiac poisonings.
Acknowledgments
We would like to acknowledge the caregivers and staff at Maine Medical Center who show up every day to make outcomes like this possible.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-22-2/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-22-2/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Larkin GL, Smith RP, Beautrais AL. Trends in US emergency department visits for suicide attempts, 1992-2001. Crisis 2008;29:73-80. [Crossref] [PubMed]
- Vo KT, Merriman AJ, Wang RC. Seizure in venlafaxine overdose: a 10-year retrospective review of the California poison control system. Clin Toxicol (Phila) 2020;58:984-90. [Crossref] [PubMed]
- Schroeder I, Zoller M, Angstwurm M, et al. Venlafaxine intoxication with development of takotsubo cardiomyopathy: successful use of extracorporeal life support, intravenous lipid emulsion and CytoSorb®. Int J Artif Organs 2017;40:358-60. [Crossref] [PubMed]
- Murphy L, Rasmussen J, Murphy NG. Venlafaxine overdose treated with extracorporeal life support. CMAJ 2021;193:E167-70. [Crossref] [PubMed]
- Marquetand C, Langer HF, Klein JP, et al. The Use of Extracorporeal Life Support in a Patient Suffering from Venlafaxine Intoxication. A Case Report. J Crit Care Med (Targu Mures) 2020;6:120-3. [Crossref] [PubMed]
- Johnson NJ, Gaieski DF, Allen SR, et al. A review of emergency cardiopulmonary bypass for severe poisoning by cardiotoxic drugs. J Med Toxicol 2013;9:54-60. [Crossref] [PubMed]
- Holliday SM, Benfield P. Venlafaxine. A review of its pharmacology and therapeutic potential in depression. Drugs 1995;49:280-94. [Crossref] [PubMed]
- National Institutes of Health. DailyMed - venlafaxine hydrochloride capsule, extended release. Retrieved September 29, 2021. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6c7c6190-b35f-4228-ba3d-2cb3149c81b3
- Vinetti M, Haufroid V, Capron A, et al. Severe acute cardiomyopathy associated with venlafaxine overdose and possible role of CYP2D6 and CYP2C19 polymorphisms. Clin Toxicol (Phila) 2011;49:865-9. [Crossref] [PubMed]
- Vasudev R, Rampal U, Patel H, et al. Selective Serotonin-norepinephrine Reuptake Inhibitors-induced Takotsubo Cardiomyopathy. N Am J Med Sci 2016;8:312-5. [Crossref] [PubMed]
- Khalifa M, Daleau P. Turgeon aJ. Mechanism of sodium channel block by venlafaxine in guinea pig ventricular myocytes. J Pharmacol Exp Ther 1999;291:280-4. [PubMed]
- Castanares-Zapatero D, Gillard N, Capron A, et al. Reversible cardiac dysfunction after venlafaxine overdose and possible influence of genotype and metabolism. Forensic Sci Int 2016;266:e48-51. [Crossref] [PubMed]
- Stefani M, Roberts DM, Brett J. High-dose insulin euglycemic therapy to treat cardiomyopathy associated with massive venlafaxine overdose. Clin Toxicol (Phila) 2020;58:299-300. [Crossref] [PubMed]
- Hillyard SG, Barrera-Groba C, Tighe R. Intralipid reverses coma associated with zopiclone and venlafaxine overdose. Eur J Anaesthesiol 2010;27:582-3. [Crossref] [PubMed]
- Dagtekin O, Marcus H, Müller C, et al. Lipid therapy for serotonin syndrome after intoxication with venlafaxine, lamotrigine and diazepam. Minerva Anestesiol 2011;77:93-5. [PubMed]
Cite this article as: Felix A, Campbell D, Neavyn M, Hackett P. Rescue veno-arterial extracorporeal membrane oxygenation in venlafaxine overdose: a case report. J Emerg Crit Care Med 2022;6:16.


