
Fast and Fusariosis: a systematic review and case report of a rapidly fatal central nervous system infection
Introduction
Fusarium is a ubiquitous fungal species commonly found in soil, water, and plants that can cause infections in immunocompetent as well as immunocompromised people. Immunocompetent patients are more likely to suffer from local skin infections, whereas immunosuppressed people are susceptible to invasive infections (1). Most Fusarium infections are caused by F. solani, F. oxysporum, and F. moniliforme (1). When infections do arise, it is most often spread via skin or respiratory tracts, which can arise as localized necrotic tissue lesions in the lungs or skin (1).
Although invasive Fusariosis is exceedingly uncommon, the prognosis is poor, with mortality approaching as high as 90%, and with many cases being from patients with hematologic malignancies or following stem cell transplants (1-4). Infections following organ transplant are even more uncommon, with no previous case following a heart transplant.
Given the rarity of this condition and the necessity for rapid diagnosis and appropriate management, we present a case of central nervous system (CNS) Fusariosis, as well as a thorough review of previously documented cases of systemic Fusariosis, as well as a discussion of available diagnostic modalities and available interventions to guide the critical care physician in managing patients with this rare condition. We present the following article in accordance with the CARE reporting checklist (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-21-125/rc).
Case presentation
A 57-year-old immunocompromised male with a previous medical history of orthotopic heart transplant secondary to ischemic cardiomyopathy one-month prior presented to the emergency department with a three-day history of unremitting generalized headache, right eye pain, and unilateral eye redness. He denied any systemic symptoms, including fever, chills, vision changes, dizziness, dysarthria, focal weakness, paresthesia, nausea, or vomiting. Physical exam was remarkable for left facial droop and ⅘ muscle strength in the left upper and lower extremity.
Laboratory studies revealed hyponatremia at 128 mEq/L and a leukocytosis of 16.3×103 cells/microL but were otherwise unremarkable. He reported being adherent to his immunosuppressive regimen, which consisted of tacrolimus, mycophenolate, and prednisone, as well as his infection prophylaxis regimen which included nystatin, valganciclovir, and trimethoprim/sulfamethoxazole. Given the focal neurologic signs, a computed tomography (CT) head and MRI brain was ordered, and demonstrated over 10 enhancing intraparenchymal lesions, the largest being in the frontoparietal region and measuring 15 mm × 13 mm (Figure 1).
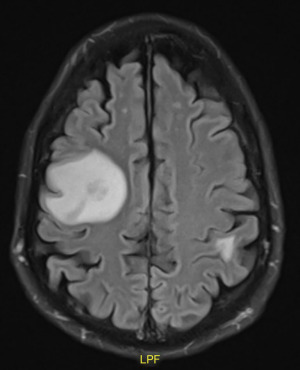
The patient was admitted for probable infectious vs. metastatic etiology, and neurosurgery, ophthalmology, and infectious disease were consulted. The patient’s eye pain was diagnosed as iritis and improved with prednisone drops. On the second day of admission, the patient developed worsening nausea and bilateral upper extremity tremors. The patient began seizing and subsequently required endotracheal intubation for airway protection. An emergent repeat CT head was performed and revealed no acute changes or intracranial bleed. Lumbar puncture revealed a clear, colorless fluid with normal opening pressure, neutrophilic pleocytosis, elevated protein (63 mg/dL) with a normal glucose level. Cerebrospinal fluid VDRL, Cryptococcus antigen, HSV-1 PCR, and HSV-2 PCR, Toxoplasma IgG, and gram stain and culture were all negative. Blood, sputum, and urine cultures were negative. A microbial cell-free DNA test (Karius© Redwood City, CA) was positive for Fusarium solani.
The patient was immediately started on intravenous amphotericin B and voriconazole, and his immunosuppressive agents were temporarily reduced. On hospital day eight, the patient’s headache and right eye pain suddenly worsened. Physical exam demonstrated right pupillary dilation prompting repeat imaging, which illustrated increased abscesses size and worsening surrounding edema. The patient underwent emergent neurosurgical drainage and biopsy, which was consistent with Fusariosis spp. The patient was also administered intravitreal antifungal therapy for the Fusarium endophthalmitis. Post-surgical repeat MRI demonstrated multiple new lesions and worsening edema (Figure 2).
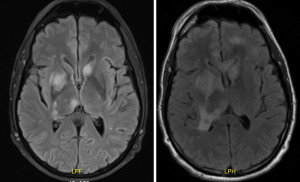
One day following the surgery, the patient demonstrated signs of acute heart failure, and underwent emergent right heart catheterization with endomyocardial biopsy that revealed acute transplant rejection. Unfortunately, given the advanced stage of his infection coupled with transplant rejection, palliative care was consulted, and the patient was discharged to hospice.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The PubMed database and all major infectious disease and critical care journals were searched during December 2021 using the keywords “Fusariosis”, “CNS”, “Invasive”, and “Disseminated”, alone or in combination to obtain articles fitting the inclusion and exclusion criteria. The inclusion criteria were CNS Fusariosis involving any region of the brain. Cases of Fusariosis not invading the brain structures, as well as non-Fusariosis fungal infections invading the CNS, were excluded.
To date, only 21 cases of CNS Fusariosis have been reported since 1974 (Table 1) (1,3,5-21). The outcomes of these cases remained poor, with only two cases noting survival following discovery and treatment of the infection. The age of discovery spanned from one to 76 years old, with no age bracket demonstrating a higher propensity for infection. However, the mean age of discovery is 37.63 years (95% CI: 25.6 to 45.6 years). The most common region of brain involvement was the cerebral cortex (Figure 3), however it can arise anywhere within the brain structures, and demonstrates little preference for any particular region. In addition, the most common neurologic disturbance was related to altered mental status; however, the most common presenting symptoms overall was related to skin and respiratory involvement, as previous studies have suggested (Table 2) (1,3,5-21).
Table 1
| Author, year | Patient age (years) | Predisposing condition | CNS location | Extra-CNS location | Presenting symptom |
|---|---|---|---|---|---|
| Abramowsky, 1974 | 2 | Burn | Cerebral cortex | Skin, kidney, heart | Skin lesion |
| Agamanolis, 1991 | 15 | Leukemia | Meninges | Skin | AMS, skin lesions |
| Anaissie, 1986 | 53 | Multiple myeloma | Not specified | Skin, blood, heart, lung, GI, kidneys | Pneumonia, skin lesions |
| Anten, 2008 | 53 | Unspecified cancer | Cerebral cortex | Lungs, skin | Fever, AMS |
| Antunes, 1998 | Not specified | Not specified | Meninges | Not specified | Not specified |
| Bleggi-Torres, 1996 | 6 | Aplastic anemia | Brainstem, cerebellum | Skin, lung | Skin lesion |
| Chen, 2017 | 65 | None | Brain stem, cerebellum | None | AMS |
| Garcia, 2015 | 50 | Leukemia | Cerebral cortex | Not specified | AMS |
| Kleinschmidt-Demasters, 2009 | 76 | Leukemia | Cerebellum | Skin | Skin lesion |
| Lortholary, 2010 | Not specified | Not specified | Not specified | Not specified | Not specified |
| Mehta, 2014 | 20 | Leukemia | Thalamus, cerebellum, cerebral cortex | Skin, blood | Fever, skin lesions |
| Muhammed, 2013 | 23 | Kidney transplant | Meninges | Blood | Nausea, vomiting, headache |
| Njambi, 2007 | 53 | Leukemia | Cerebral cortex | Not specified | Not specified |
| Nucci, 2003 | 55 | Lung transplant | Not specified | Skin, lung, intestines, kidney | Skin lesion |
| Schwartz, 2015 | 1 | Aplastic anemia | Not specified | Skin, lung, blood | Skin lesions |
| Schwartz, 2015 | 15 | Leukemia | Cerebral cortex | Lung | Respiratory distress, hemoptysis |
| Steinberg, 1983 | 17 | Infectious mononucleosis | Brain stem | Skin | Gastrointestinal distress, skin lesion |
| Stropnicky, 2021 | 65 | Liver transplant | Cerebral cortex, cerebellum, brain stem | None | Pupillary difference |
| Turra, 2020 | 13 | Leukemia | Not specified | Skin, blood | Skin lesions, facial paralysis, AMS |
| Vadhan, 2020 (present case) | 57 | Heart transplant | Cerebral cortex | Eye | Headache, eye pain |
| Vincent, 2003 | 76 | Leukemia | Cerebellum | Skin | Skin lesion |
CNS, central nervous system; AMS, altered mental status; GI, gastrointestinal.
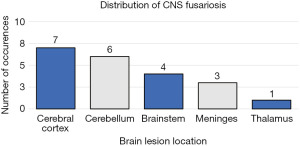
Table 2
| Presenting symptom | Incidence |
|---|---|
| Skin lesion | 11 |
| AMS | 5 |
| Respiratory distress | 2 |
| GI distress | 2 |
| Fever | 2 |
| Facial paralysis | 1 |
| Headache | 1 |
| Eye pain | 1 |
| Pupillary changes | 1 |
AMS, altered mental status; GI, gastrointestinal.
The clinical presentation of infection with Fusarium is vast, ranging from localized infection of the skin, nails, or eyes in immunocompetent individuals, to invasive and disseminated forms in the immunocompromised. Sites of invasive infection most often include the skin, eyes, lungs, and sinuses; less commonly involved are the bones, joints, and such as this case, the brain (2,4,22). Skin manifestations typically present as multiple painful erythematous nodules with centralized necrosis (via angioinvasion), resembling a target-like appearance (4). Given the prevalence of skin-derived Fusariosis among systemic disease, a thorough dermatologic examination is highly recommended, carefully assessing for the presence of skin or nail infections. Following skin involvement, the respiratory system is the second most common gateway for systemic infection (22). One final unique characteristic of invasive Fusariosis compared to other CNS fungal infections is its higher incidence of ophthalmologic related symptoms, including endophthalmitis or chorioretinitis, as was the case in our patient (23).
The gold standard diagnosis of Fusariosis is tissue culture. Blood cultures in patients with invasive Fusariosis are limited, with only 60% sensitivity (11,24). Paradoxically, an Aspergillus galactomannan antigen assay can detect Fusarium infections, however given the clinical similarities between Aspergillus and Fusarium spp., this may be a source of confusion, despite having similar treatment strategies (24). More recently, the utilization of cell-free DNA (cfDNA) plasma extraction with next-generation sequencing has enabled rapid, accurate, and noninvasive testing for invasive fungal infections (25). For this patient, cfDNA was used to confirm the diagnosis.
Treating invasive Fusariosis remains a clinical challenge due to the high drug resistance rate, which contributes to its high mortality rate (4). Nonetheless, invasive management involves a two-tailed approach: first, voriconazole and amphotericin B, or Posaconazole should be administered (4,26,27). Second, the patient’s innate immunocompromised status (specifically neutropenia) must be reversed, as the most important risk factor for contracting invasive Fusariosis and the overall prognosis is the neutropenic status (4,11,23,28). Unfortunately, data to support the use of granulocyte colony-stimulating factor and granulocyte transfusions in these patients is limited (28).
In patients at risk of herniation, emergent surgical resection is recommended and can alleviate compression and control infection spread; however, its impact on clinical outcomes is inconclusive (4). Unfortunately, regardless of therapeutic interventions, the outcome is nearly always fatal if neutrophil recovery is not achieved (1). A summary of recommendations for the diagnostic workup and available treatment strategies for invasive Fusariosis can be seen in Nucci et al., 2007 (2). It is important to note that our patient was not neutropenic, further emphasizing the severity of this aggressive infection.
Conclusions
Patients with invasive Fusariosis most commonly have a history of immunocompromised status and typically present with dermatologic, respiratory, or as in this case, ophthalmologic symptoms. Unfortunately, despite aggressive treatment with potent systemic antifungal therapies and neurosurgical resection, clinical outcomes remain poor. Given this, physicians must maintain a high index of suspicion for CNS Fusariosis among immunocompromised individuals because delay in diagnosis and treatment will almost assuredly result in catastrophic consequences. In addition, given the paucity of reported cases and lack of positive outcomes, we urge further research regarding CNS Fusariosis with the goal of improving treatment outcomes of this devastating disease.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-21-125/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-21-125/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stropnicky P, Heß K, Becker T, et al. Disseminated cerebral fusariosis in a liver-transplant patient: A case report and review of the literature. Z Gastroenterol 2021; Epub ahead of print. [Crossref] [PubMed]
- Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev 2007;20:695-704. [Crossref] [PubMed]
- Agamanolis DP, Kalwinsky DK, Krill CE Jr, et al. Fusarium meningoencephalitis in a child with acute leukemia. Neuropediatrics 1991;22:110-2. [Crossref] [PubMed]
- Tortorano AM, Richardson M, Roilides E, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect 2014;20:27-46. [Crossref] [PubMed]
- Abramowsky CR, Quinn D, Bradford WD, et al. Systemic infection by fusarium in a burned child. The emergence of a saprophytic strain. J Pediatr 1974;84:561-4. [Crossref] [PubMed]
- Anaissie E, Kantarjian H, Jones P, et al. Fusarium. A newly recognized fungal pathogen in immunosuppressed patients. Cancer 1986;57:2141-5. [Crossref] [PubMed]
- Anten S, Heddema ER, Visser O, et al. Images in haematology. Cerebral fungal abscess in a patient with acute promyelocytic leukaemia. Br J Haematol 2008;140:253. [Crossref] [PubMed]
- Antunes NL, Hariharan S, DeAngelis LM. Brain abscesses in children with cancer. Med Pediatr Oncol 1998;31:19-21. [Crossref] [PubMed]
- Bleggi-Torres LF, de Medeiros BC, Neto JZ, et al. Disseminated Fusarium sp. infection affecting the brain of a child after bone marrow transplantation. Bone Marrow Transplant 1996;18:1013-5. [PubMed]
- Chen YJ, Chou CL, Lai KJ, et al. Fusarium Brain Abscess in a Patient with Diabetes Mellitus and Liver Cirrhosis. Acta Neurol Taiwan 2017;26:128-32. [PubMed]
- Garcia RR, Min Z, Narasimhan S, et al. Fusarium brain abscess: case report and literature review. Mycoses 2015;58:22-6. [Crossref] [PubMed]
- Kleinschmidt-Demasters BK. Disseminated Fusarium infection with brain abscesses in a lung transplant recipient. Clin Neuropathol 2009;28:417-21. [Crossref] [PubMed]
- Lortholary O, Obenga G, Biswas P, et al. International retrospective analysis of 73 cases of invasive fusariosis treated with voriconazole. Antimicrob Agents Chemother 2010;54:4446-50. [Crossref] [PubMed]
- Mehta A, Bellam N. Disseminated fusariosis during acute myelogenous leukemia induction treatment. Blood 2014;123:3379. [Crossref] [PubMed]
- Muhammed M, Anagnostou T, Desalermos A, et al. Fusarium infection: report of 26 cases and review of 97 cases from the literature. Medicine (Baltimore) 2013;92:305-16. [Crossref] [PubMed]
- Njambi S, Huttova M, Kovac M, et al. Fungal neuroinfections: rare disease but unacceptably high mortality. Neuro Endocrinol Lett 2007;28:25-6. [PubMed]
- Nucci M. Emerging moulds: Fusarium, Scedosporium and Zygomycetes in transplant recipients. Curr Opin Infect Dis 2003;16:607-12. [Crossref] [PubMed]
- Schwartz KL, Sheffield H, Richardson SE, et al. Invasive Fusariosis: A Single Pediatric Center 15-Year Experience. J Pediatric Infect Dis Soc 2015;4:163-70. [Crossref] [PubMed]
- Steinberg GK, Britt RH, Enzmann DR, et al. Fusarium brain abscess. Case report. J Neurosurg 1983;58:598-601. [Crossref] [PubMed]
- Turra N, Acosta A, Incoronato A, et al. Multisystemic fusariosis with fulminant evolution. An Bras Dermatol 2020;95:645-8. [Crossref] [PubMed]
- Vincent AL, Cabrero JE, Greene JN, et al. Successful voriconazole therapy of disseminated Fusarium solani in the brain of a neutropenic cancer patient. Cancer Control 2003;10:414-9. [Crossref] [PubMed]
- Batista BG, Chaves MA, Reginatto P, et al. Human fusariosis: An emerging infection that is difficult to treat. Rev Soc Bras Med Trop 2020;53:e20200013. [Crossref] [PubMed]
- McCarthy M, Rosengart A, Schuetz AN, et al. Mold infections of the central nervous system. N Engl J Med 2014;371:150-60. [Crossref] [PubMed]
- Tortorano AM, Esposto MC, Prigitano A, et al. Cross-reactivity of Fusarium spp. in the Aspergillus Galactomannan enzyme-linked immunosorbent assay. J Clin Microbiol 2012;50:1051-3. [Crossref] [PubMed]
- Hong DK, Blauwkamp TA, Kertesz M, et al. Liquid biopsy for infectious diseases: sequencing of cell-free plasma to detect pathogen DNA in patients with invasive fungal disease. Diagn Microbiol Infect Dis 2018;92:210-3. [Crossref] [PubMed]
- Al-Hatmi AMS, Bonifaz A, Ranque S, et al. Current antifungal treatment of fusariosis. Int J Antimicrob Agents 2018;51:326-32. [Crossref] [PubMed]
- Ortoneda M, Capilla J, Pastor FJ, et al. Efficacy of liposomal amphotericin B in treatment of systemic murine fusariosis. Antimicrob Agents Chemother 2002;46:2273-5. [Crossref] [PubMed]
- Kadri SS, Remy KE, Strich JR, et al. Role of granulocyte transfusions in invasive fusariosis: systematic review and single-center experience. Transfusion 2015;55:2076-85. [Crossref] [PubMed]
Cite this article as: Vadhan JD, Melo AJ, Shogan JC, Singh V, Carrillo M. Fast and Fusariosis: a systematic review and case report of a rapidly fatal central nervous system infection. J Emerg Crit Care Med 2022;6:24.


