
Efficacy and safety of propofol dosing based on ideal body weight vs. actual body weight: a retrospective cohort study
Introduction
Propofol is a highly lipophilic sedative-hypnotic that causes central nervous system (CNS) depression due to its positive modulation of gamma aminobutyric acid (GABA) and inhibition of N-Methyl-D-aspartate (NMDA) (1-3). The Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption (PADIS) guidelines by the Society of Critical Care Medicine (SCCM) recommend propofol as a preferred agent for sedation (4). Propofol is widely used due to its favorable pharmacokinetics, with a short onset of 40 seconds and duration of action of 3 to 10 minutes (1). After long-term infusions, the terminal half-life of propofol can increase to more than 48 hours, though the clinical effect is much shorter (3). Nonetheless, variations in pharmacokinetics (PK) and pharmacodynamics (PD) can lead to differences in incidence of adverse drug reactions, drug accumulation, and time to target sedation goal.
Various dosing weight strategies for propofol infusion have been assessed (5). Actual body weight (ABW)-based dosing was found to be appropriate in non-obese patients, but in obese patients it can potentially lead to more adverse effects because the proportion of lean body weight (LBW) does not increase to the same extent as adipose tissue. Thus, giving a weight-based dose of propofol in obese patients will increase the drug concentration to lean tissue, and therefore contribute to a greater effect on the brain and heart. Ideal body weight (IBW) only takes height and sex into consideration, so it will lead to lower dose of propofol and less drug accumulation over time (5). LBW differs by taking body fat into consideration and has been found to be the body size descriptor that best captures the needs of propofol dosing in the setting of procedures (6,7). These studies show that a patient’s body composition can affect the success of the dosing strategy, but there are no head-to-head comparisons assessing the efficacy and safety of the different weight-based dosing strategies.
During the peak of the first COVID-19 pandemic wave in 2020, our Health System made the decision to change the default dosing weight of propofol infusion to IBW as a drug conservation strategy. The purpose of this study is to compare the efficacy and safety of ABW vs. IBW dosing strategies of propofol infusion in mechanically ventilated adult patients in the intensive care units (ICUs) of a large academic health system. We present the following article in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting checklist (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-21-115/rc).
Methods
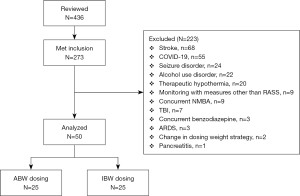
This is a multicenter, institutional review board approved, retrospective cohort study at NYU Langone Health (NYULH), a large academic health system consisting of 4 hospitals, from January 2019 to November 2020. This study aimed to compare patients receiving a continuous infusion of propofol utilizing ABW with those dosed by IBW. The ABW-based dosing group was selected in the timeframe from January to December 2019 and the IBW-based group from June 2020 to November 2020. All adult patients that received at least 24 hours of a continuous propofol infusion in the ICU while on mechanical ventilation were eligible for screening. Exclusion criteria included patients whose depth of sedation was assessed using a scale other than Richmond Agitation and Sedation Scale (RASS), change in dosing weight strategy during propofol administration, dosing based on adjusted body weight (AdjBW), patients receiving concurrent neuromuscular blocking agents (NMBA) or benzodiazepine infusions. Patients were also excluded if they had an underlying medical condition that may blunt the ability to assess depth of sedation or preclude them from receiving propofol including pancreatitis, familial combined hyperlipidemia (FCHL), seizure disorder, alcohol use disorder, therapeutic hypothermia, traumatic brain injury (TBI), stroke, COVID-19, and acute respiratory distress syndrome (ARDS).
Electronic health records (EHRs) were retrospectively reviewed for baseline demographics, pertinent medical history, reason for ICU admission, and a sequential organ failure assessment (SOFA) score at the start of propofol infusion. Data up to 72 hours from propofol infusion initiation were collected, including propofol boluses required, median infusion rates and titrations to target RASS as well as daily requirements of adjunct analgesia, sedation and vasoactive medication utilization. Other data collected included pertinent laboratory values such as triglycerides, creatinine kinase, initial and daily RASS scores.
Sedation and analgesia for patients receiving mechanical ventilation is managed at the discretion of the attending physician. Fentanyl is the preferred analgesic and is often used as a first-line agent to lower sedative requirements. Propofol and dexmedetomidine are our preferred sedatives, often used in combination with fentanyl. Continuous infusions of benzodiazepines are avoided unless patients cannot tolerate other sedative agents. Sedatives and analgesics are titrated by our nurses at the bedside based on a determined RASS goal, electronic medication order, and nursing titration guide (Appendix 1). Light sedation is defined as a target RASS of 0 to −2, and deep sedation is defined as target RASS of less than −2.
The primary outcome was time to target RASS (as indicated in the initial order) from propofol infusion initiation. Secondary endpoints were propofol infusion rates and boluses required to reach target RASS, propofol titrations, time within RASS goal for up to 72 hours from propofol infusion initiation, concomitant sedative needs, time to extubation, rate of re-intubations, duration of mechanical ventilation, ICU and hospital lengths of stay, and in-hospital mortality. Safety endpoints included clinically significant hypotension, hypertriglyceridemia (triglycerides >500 mg/dL), bradycardia (heart rate <50 beats per minute) and propofol-related infusion syndrome. Clinically significant hypotension was defined as new initiation or increase in vasopressor requirements. Propofol related infusion syndrome was defined as acute refractory bradycardia leading to asystole in the presence of one or more of the following: metabolic acidosis (base excess of −10 mmol/L), hyperkalemia (K >5.5 mmol/L), rhabdomyolysis (creatinine kinase >5,000 units/L) or myoglobinuria, renal failure, lipemic plasma, or fatty liver enlargement (8).
All data was secure, and statistics were conducted by the investigators using SPSS Statistics Software (IBM Corp., Armonk, NY, USA; version 25.0). Descriptive variables were reported as median [interquartile range (IQR)], unless otherwise noted. Categorical variables were described as frequencies or proportions and compared using Chi square test or Fisher’s exact test. Continuous variables were described as medians with IQRs and analyzed using Mann-Whitney U test. A two-sided alpha value of <0.05 was considered to be statistically significant.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by New York University (NYU) Langone Health Institutional Review Board (No. s20-02067) and individual consent for this retrospective analysis was waived.
Results
After screening 436 patients, 50 patients were included in the final analysis. Screening methods and reasons for exclusion are summarized in Figure 1. Of the 50 patients included, 25 patients used IBW and 25 patients used ABW as dosing weights for their respective propofol continuous infusions.
The median age in our cohort was 66 years with 60% males. There were no significant differences in past medical history or prior to admission medications as displayed in Table 1. The dosing weights were significantly lower in the IBW group as compared to the ABW group (61.5 vs. 81 kg, P=0.001). A majority of patients in the IBW group were overweight with a body mass index (BMI) 25–29 kg/m2 (52% vs. 20%, P=0.018), while there was no difference between groups in patients with BMI 30–39 kg/m2 (32% vs. 36%, P=0.765) or BMI ≥40 kg/m2 (4% vs. 12%, P=0.609). Acute hypoxic respiratory failure was the most common ICU admission diagnosis (60%) in both groups, however, the IBW group had a significantly higher rate of admission for sepsis (36% vs. 4%, P=0.005). The median SOFA scores were 11 and 10 in the IBW vs. ABW group, respectively (P=0.220). Similar number of sedatives and doses of sedatives and vasopressors prior to propofol initiation were used between groups. Prior to propofol infusion, patients most commonly required norepinephrine as a vasopressor (44% vs. 40%) at a similar median dose on day 1 (0.04 vs. 0.065 mcg/kg/min, P=0.686). Twenty-six percent of patients were on sedatives prior to propofol infusion, most commonly fentanyl and dexmedetomidine at median rates of 50 mcg/kg/h and 0.9 mcg/kg/h, respectively. The IBW group targeted a deeper initial RASS goal (−2 vs. −1, P=0.004).
Table 1
| Baseline characteristics | Total (n=50) | IBW (n=25) | ABW (n=25) | P value |
|---|---|---|---|---|
| Female | 20 [40] | 8 [32] | 12 [48] | 0.248 |
| Age (years), median [IQR] | 66 [56–76] | 67 [58–77] | 65 [56–75] | 0.509 |
| Race | ||||
| White | 27 [54] | 13 [52] | 14 [56] | 0.777 |
| Black | 5 [10] | 2 [8] | 3 [12] | 1.00 |
| Other | 18 [36] | 10 [40] | 8 [32] | 0.556 |
| Actual weight, median [IQR] (kg) | 78.5 [70–92] | 77 [71–89] | 81 [70–95] | 1.00 |
| Actual weight method | ||||
| Scale | 25 [50] | 9 [36] | 16 [64] | 0.048 |
| Estimated | 16 [32] | 9 [36] | 7 [28] | 0.066 |
| Stated | 9 [18] | 7 [28] | 2 [8] | 0.544 |
| BMI, median [IQR] (kg/m2) | 28.4 [25–33] | 27.5 [26–31] | 29.2 [24–35] | 0.938 |
| Overweight (25–<30) | 18 [36] | 13 [52] | 5 [20] | 0.018 |
| Obese (30–<40) | 17 [34] | 8 [32] | 9 [36] | 0.765 |
| Morbidly obese (≥40) | 4 [8] | 1 [4] | 3 [12] | 0.609 |
| Propofol dosing weight, median [IQR] (kg) | 69.3 [57–80] | 61.5 [53–68] | 81 [70–95] | 0.001 |
| Past medical history | ||||
| Hypertension | 30 [60] | 13 [52] | 17 [68] | 0.248 |
| Hyperlipidemia | 24 [48] | 11 [44] | 13 [52] | 0.571 |
| Diabetes | 15 [30] | 6 [24] | 9 [36] | 0.355 |
| Coronary artery disease | 13 [26] | 5 [20] | 8 [32] | 0.333 |
| Heart failure | 9 [18] | 3 [12] | 6 [24] | 0.269 |
| Ejection fraction, %, median [IQR] | 55 [50–65] | 55 [50–65] | 55 [45–65] | 0.696 |
| Chronic kidney disease | 7 [14] | 5 [20] | 2 [8] | 0.221 |
| Liver disease | 4 [8] | 2 [8] | 2 [8] | 0.695 |
| COPD | 12 [24] | 5 [20] | 7 [28] | 0.508 |
| Cancer | 14 [28] | 6 [24] | 8 [32] | 0.529 |
| IV drug abuse | 2 [4] | 2 [8] | – | 0.490 |
| Prior to admission benzodiazepine use | 6 [12] | 2 [8] | 4 [16] | 0.384 |
| Prior to admission opioid use | 4 [8] | 3 [12] | 1 [4] | 0.297 |
| Prior to admission GABAergic medication use | 6 [12] | 2 [8] | 4 [16] | 0.384 |
| SOFA score on ICU admission, median [IQR] | 10 [9–13] | 11 [9–14] | 10 [9–12] | 0.220 |
| ICU primary problem | ||||
| Infection/sepsis | 10 [20] | 9 [36] | 1 [4] | 0.005 |
| Acute hypoxic respiratory failure | 30 [60] | 12 [48] | 18 [72] | 0.083 |
| Cardiovascular | 6 [12] | 2 [8] | 4 [16] | 0.667 |
| Other | 4 [8] | 2 [8] | 2 [8] | 1.00 |
| Initial sedatives | 13 [26] | 5 [20] | 8 [32] | 0.333 |
| Fentanyl | 7 [54] | 2 [40] | 5 [63] | 0.417 |
| Dose, median [IQR] (mcg/h) | 50 [50–100] | 50 [50–50] | 50 [50–150] | 0.669 |
| Dexmedetomidine | 10 [77] | 4 [80] | 6 [75] | 0.480 |
| Dose, median [IQR] (mcg/kg/h) | 0.9 [1–1] | 1 [1–1] | 0.9 [1–1] | 0.745 |
| Initial vasopressors | 14 [28] | 6 [24] | 8 [32] | 0.529 |
| Norepinephrine dose, median [IQR] (mcg/kg/min) | 0.04 [0.05–0.25] | 0.04 [0.01–0.25] | 0.065 [0.05–0.15] | 0.686 |
| Initial starting dose of propofol, median [IQR] (mcg/kg/min) | 20 [10–30] | 15 [10–28] | 20 [10–30] | 0.473 |
| Initial goal RASS, median [IQR] | −2 [−3, −1] | −2 [−3, −2] | −1 [−2, −1] | 0.004 |
| 0 | 6 [12] | 1 [4] | 5 [20] | 0.189 |
| −1 | 8 [16] | – | 8 [32] | 0.004 |
| −2 | 20 [40] | 14 [56] | 6 [24] | 0.021 |
| −3 | 10 [20] | 5 [20] | 5 [20] | 1.00 |
| −4 | 6 [12] | 5 [20] | 1 [4] | 0.189 |
| Light sedation (RASS goal 0 to −2) | 34 [68] | 15 [60] | 19 [76] | 0.225 |
All values are expressed as n [%] unless otherwise noted. IBW, ideal body weight; ABW, actual body weight; IQR, interquartile range; BMI, body mass index; COPD, chronic obstructive pulmonary disease; IV, intravenous; SOFA, Sequential Organ Failure Assessment; ICU, intensive care unit; RASS, Richmond Agitation Sedation Scale.
The primary outcome of time to target RASS did not demonstrate a significant difference based on the dosing weight utilized for propofol infusions (IBW: 6 hours vs. ABW: 7 hours, P=0.714) (Figure 2). In addition, 8 (32%) patients in the IBW and 6 (24%) patients in the ABW group did not achieve their respective goal RASS within the 72-hour timeframe of this analysis (Table 2). Of those who did not meet their sedation goal, 64% were over-sedated, 28% were under-sedated, and 7% patients had a documented RASS fluctuating above and below their target during the duration of the propofol infusion. No difference was observed between these groups in patients who did not meet their target RASS score.
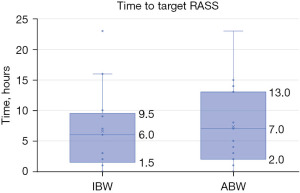
Table 2
| Outcomes | IBW (n=25) | ABW (n=25) | P value |
|---|---|---|---|
| Goal RASS reached within 72 hours, n [%] | 17 [68] | 19 [76] | 0.529 |
| Time to goal RASS, hours | 6 [2–10] | 7 [3–13] | 0.714 |
| Goal RASS not reached within 72 hours, n [%] | 8 [32] | 6 [24] | 0.529 |
| Over-sedated RASS compared to target, n [%] | 4 [29] | 5 [36] | 0.659 |
| Under-sedated RASS compared to target, n [%] | 4 [29] | – | 0.085 |
| Fluctuating RASS compared to target, n [%] | – | 1 [7] | 0.429 |
| Propofol bolus, n [%] | 7 [28] | 2 [8] | 0.138 |
| Propofol bolus dose, mcg | 50 [30–75] | 35 [28–43] | 0.077 |
| Propofol dose to achieve goal RASS, mcg/kg/min | 30 [20–40] | 30 [10–40] | 0.509 |
| Propofol dose to achieve goal RASS, mg/min | 1.914 [1–2] | 1.94 [1–3] | 0.883 |
| Time from discontinuation of propofol to extubation, hours | 25.3 [14–113] | 20.8 [5–84] | 0.058 |
| Duration of mechanical ventilation, days | 5 [2–7] | 4 [3–7] | 0.520 |
| Duration of propofol, hours | 46 [28–82] | 70 [47–80] | 0.264 |
| Re-intubations, n [%] | 2 [8] | 6 [24] | 0.247 |
| ICU LOS, days | 8 [3–13] | 10 [8–16] | 0.186 |
| Hospital LOS, days | 17 [9–30] | 19 [10–24] | 0.938 |
| Mortality, n [%] | 5 [20] | 6 [24] | 0.733 |
All values are expressed as median [IQR] unless otherwise noted. IBW, ideal body weight; ABW, actual body weight; RASS, Richmond Agitation Sedation Scale; ICU, intensive care unit; LOS, length of stay; IQR, interquartile range.
Outcomes were assessed in patients who achieved their target RASS goal. In the time to achieve patient-specific sedation goals, the median weight-based (30 vs. 30 mcg/kg/min, P=0.509) and median total propofol doses (1.914 vs. 1.94 mg/min, P=0.883) were similar between groups. The number of propofol bolus doses (7 vs. 2, P=0.138) administered per patient and the median bolus dose (50 vs. 35 mcg, P=0.077), were not significantly different amongst the groups. Supplemental sedation to achieve sedation goals, were provided with continuous fentanyl and dexmedetomidine infusions (Table 3). There was no difference between IBW and ABW groups in median fentanyl (50 vs. 100 mcg/h, P=0.974) or dexmedetomidine requirements (1.15 vs. 1.5 mcg/kg/h, P=0.362) to reach a goal RASS. There was no difference in total propofol titrations (5 vs. 3.5, P=0.353), up titrations (60% vs. 45.4%, P=0.594) or down titrations (33.3% vs. 20%, P=0.906) in order to achieve target RASS (Table 4). Between IBW and ABW groups, there was no difference in the percentage of documented RASS scores at goal (46.7% vs. 54.5%, P=0.220).
Table 3
| Variable | IBW (n=17) | ABW (n=19) | P value |
|---|---|---|---|
| Fentanyl infusion, n [%] | 11 [65] | 10 [53] | 0.765 |
| Infusion rate, mcg/h | 50 [38–138] | 100 [50–138] | 0.974 |
| Dexmedetomidine infusion, n [%] | 4 [24] | 2 [11] | 0.226 |
| Infusion rate, mcg/kg/h | 1.15 [0.9–1.35] | 1.5 [1.25–1.75] | 0.362 |
All values are expressed as median [IQR] unless otherwise noted. RASS, Richmond Agitation Sedation Scale; IBW, ideal body weight; ABW, actual body weight.
Table 4
| Variable | IBW (n=17) | ABW (n=19) | P value |
|---|---|---|---|
| Total titrations | 5 [2–7] | 3.5 [1–5] | 0.353 |
| Up titrations, % | 60 [43–71] | 45.4 [10–82] | 0.594 |
| Down titrations, % | 33.3 [0–50] | 20 [0–57] | 0.906 |
| Documented RASS scores | 20 [13–32] | 21 [13–32] | 0.741 |
| Time in RASS goal, % | 46.7 [15–57] | 54.5 [40–68] | 0.220 |
All values are expressed as median [IQR]. RASS, Richmond Agitation Sedation Scale; IBW, ideal body weight; ABW, actual body weight; IQR, interquartile range.
Other outcomes were assessed after achieving sedation goals during the 72-hour follow up period. There was no difference in the total duration of propofol between groups (46 vs. 70 hours, P=0.264), time from propofol discontinuation to extubation (25.3 vs. 20.8 hours, P=0.058), duration of mechanical ventilation (5 vs. 7 days, P=0.520), re-intubations (8% vs. 24%, P=0.247), ICU length of stay (8 vs. 10 days, P=0.186), hospital length of stay (17 vs. 19 days, P=0.938), or mortality (20% vs. 24%, P=0.733) (Table 2). Bradycardia, hypertriglyceridemia, and clinically significant hypotension as defined by new initiation of vasopressors (77% vs. 63%, P=0.774) or requiring increase in vasopressors (15% vs. 13%, P=0.972) did not differ between the groups within 72 hours of propofol administration (Table 5). Other concomitant intermittent medications during the follow up period were recorded by day, with similar rates and doses of sedative infusions, intermittent opioids, antipsychotic medications and concurrent benzodiazepines between groups (Table 6).
Table 5
| Safety outcome | IBW | ABW | P value |
|---|---|---|---|
| Bradycardia (HR ≤50 bpm), n [%] | 1 [4] | 1 [4] | 1.000 |
| Triglycerides (>500 mg/dL), n [%] | 3 [12] | – | 0.235 |
| Requiring initiation of vasopressors, n [%] | 10 [77] | 11 [63] | 0.774 |
| Requiring increase in vasopressors, n [%] | 2 [15] | 3 [13] | 0.972 |
| Requiring vasoactive agents, n [%] | 13 [52] | 16 [64] | 0.390 |
| Norepinephrine | 11 [44] | 10 [40] | |
| Vasopressin | 5 [20] | 2 [8] | |
| Phenylephrine | 1 [4] | 4 [16] | |
| Epinephrine | – | 1 [4] | |
| Dobutamine | – | 3 [12] | |
| Median vasopressor requirements, norepinephrine equivalents (mcg/kg/min) | |||
| Day 1 | 0.04 [0.01–0.25] | 0.065 [0.05–0.15] | 0.686 |
| Day 2 | 0.33 [0.17–0.59] | 0.06 [0.02–0.1] | 0.148 |
| Day 3 | 0.06 [0.06–0.11] | 0.035 [0.02–0.04] | 0.167 |
| Number vasopressors required, median [IQR] | 1 [1–1] | 1 [1–1] | 0.301 |
| Requiring 1 vasopressor | 11 [44] | 12 [44] | 0.777 |
| Requiring 2 vasopressors | 2 [8] | 3 [8] | 1.000 |
| Requiring 2+ vasopressors | – | 1 [4] | 1.000 |
All values are expressed as median [IQR] unless otherwise noted. IBW, ideal body weight; ABW, actual body weight; HR, heart rate; IQR, interquartile range.
Table 6
| Variable | IBW (n=25) | ABW (n=25) |
|---|---|---|
| Fentanyl maximal infusion rate (mcg/h) | ||
| Day 1, n [%], median dose | 11 [44], 150 [88–200] | 12 [48], 137.5 [50–181] |
| Day 2, n [%], median dose | 13 [52], 100 [75–150] | 17 [68], 100 [50–150] |
| Day 3, n [%], median dose | 8 [32], 112.5 [81–231] | 10 [40], 112.5 [100–150] |
| Dexmedetomidine maximal infusion rate (mcg/kg/h) | ||
| Day 1, n [%], median dose | 5 [20], 1.2 [0.6–1.3] | 6 [20], 1.5 [1.05–1.5] |
| Day 2, n [%], median dose | 5 [20], 0.9 [0.6–1.1] | 6 [20], 1.25 [1.0–1.5] |
| Day 3, n [%], median dose | 2 [8], 1.05 [0.83–1.28] | 3 [8], 1.5 [1.25–1.5] |
| Ketamine, n [%] (mg/kg/h) | 1 [4] | 1 [4] |
| Day 1, n [%], median dose | –, – | 1 [4], 0.5 [0.5–0.5] |
| Day 2, n [%], median dose | –, – | –, – |
| Day 3, n [%], median dose | 1 [4], 0.3 [0.3–0.3] | – |
| Intermittent opioids (fentanyl equivalents) (mcg) | ||
| Day 1, n [%], median dose | 7 [28], 100 [88–181] | 7 [28], 100 [73–100] |
| Day 2, n [%], median dose | 6 [24], 50 [38–81] | 3 [12], 50 [43–163] |
| Day 3, n [%], median dose | 5 [20], 60 [23–94] | 1 [4], 75 [75–75] |
| Antipsychotic medications | 6 [12] | 3 [12] |
| Day 1, n [%] | 3 [12] | 1 [4] |
| Haloperidol | 1 [4] | – |
| Olanzapine | 2 [8] | – |
| Quetiapine | 1 [4] | – |
| Valproic acid | – | – |
| Risperidone | – | 1 [4] |
| Day 2, n [%] | 1 [4] | 2 [8] |
| Haloperidol | – | – |
| Olanzapine | 1 [4] | – |
| Quetiapine | – | 1 [4] |
| Valproic acid | – | – |
| Risperidone | – | 1 [4] |
| Day 3, n [%] | 1 [4] | 2 [8] |
| Haloperidol | – | 1 [4] |
| Olanzapine | – | – |
| Quetiapine | 1 [4] | 1 [4] |
| Valproic acid | – | – |
| Risperidone | – | – |
| Concurrent benzodiazepines, n [%] | 1 [2] | – |
All values are expressed as median [IQR] unless otherwise noted. RASS, Richmond Agitation Sedation Scale; IBW, ideal body weight; ABW, actual body weight; IQR, interquartile range.
Given the significantly deeper target RASS goal and potential for higher propofol requirements in the IBW group at baseline, a subgroup analysis of patients targeting light sedation (RASS 0 to −2) was conducted to match cohorts. This subgroup totaled 34 patients, with 15 patients in IBW and 19 patients in ABW group. In patients targeting light sedation (RASS 0 to −2) there was no difference in time to goal RASS (6 vs. 7 hours, P=0.910). There was also no significant difference in requirements of sedation using fentanyl (73% vs. 85%, P=0.431) or dexmedetomidine (27% vs. 13%, P=0.630) nor median infusion rates of fentanyl (125 vs. 125 mcg/h, P =0.494) or dexmedetomidine (1.0 vs. 1.0 mcg/kg/h) to achieve target RASS. Patients required a similar number of total titrations between groups (6 vs. 4, P=0.918) with no significant difference in rate of up titrations (50% vs. 46.7%, P=0.764) or down titrations (33.3% vs. 50%, P=0.435). Within 72 hours after propofol infusion, there was no difference in time spent in target RASS goal between groups (46.7% vs. 61.5%, P=0.822) (Table 7).
Table 7
| Variable | IBW (n=15) | ABW (n=19) | P value |
|---|---|---|---|
| Light sedation (RASS goal 0 to −2), n/N [%] | 15 [60] | 19 [76] | 0.225 |
| Time to goal RASS | 6 [2–10] | 7 [2–13] | 0.910 |
| Fentanyl infusion, n [%] | 8 [73] | 5 [85] | 0.431 |
| Infusion rate, mcg/h | 125 [25–150] | 125 [75–150] | 0.494 |
| Dexmedetomidine infusion, n [%] | 4 [27] | 2 [13] | 0.630 |
| Infusion rate, mcg/kg/h | 1.0 [0.8–1.2] | 1.0 [1.0–1.0] | 0.424 |
| Total titrations | 6 [2–8] | 4 [2–5] | 0.918 |
| Up titrations, % | 50 [21–69] | 46.7 [40–100] | 0.764 |
| Down titrations, % | 33.3 [0–50] | 50 [0–60] | 0.435 |
| Documented RASS scores | 22 [13–33] | 17 [12–32] | 0.945 |
| Time in RASS goal, % | 46. 7 [18–58] | 61.5 [43–75] | 0.822 |
All values are expressed as median [IQR] unless otherwise noted. IBW, ideal body weight; ABW, actual body weight; RASS, Richmond Agitation Sedation Scale.
Discussion
During the COVID-19 pandemic in the spring of 2020, there was an unexpected surge in the use of high dose sedatives in critically ill patients requiring mechanical ventilation. As a medication conservation method the dosing weight of propofol was adjusted to IBW from ABW in the computerized physician order entry system at our Health System. To our knowledge, this study is the first study to compare ABW- and IBW-based dosing in patients receiving propofol infusion finding no difference in time to target RASS.
Based on its three-compartment model pharmacokinetics, propofol accumulates in adipose tissue and has an extended half-life with prolonged infusions. This was confirmed by Araújo and colleagues who found significantly higher propofol concentrations in obese patients after ABW-based dosing for induction (9). In addition, a retrospective study looking at differences between dosing requirements in obese vs. non-obese patients found that there were lower dose requirements of propofol to achieve target sedation in obese patients (10,11). Studies evaluating dosing weight strategies other than ABW are limited, and have typically used LBW for induction dosing of propofol. These small studies have found that the dosing weight of propofol is not proportional to ABW, but instead has a stronger relationship to LBW or BMI (6,11,12). However, LBW requires measurement of body fat percentage which is not typically done in the inpatient setting. Thus, LBW dosing is not as practical of a dosing strategy as ABW, IBW, or AdjBW, which are based on weighing methods or gender and height.
Based on the findings from this study, using IBW as a default dosing strategy for propofol as compared to ABW found no appreciable differences in clinical outcomes of efficacy and safety. In our patient population with a median BMI of 28.4 kg/m2, all patients in the IBW group had a statistically significant lower dosing weight than the ABW group. This would lead to a potentially lower dose of propofol administered in the IBW group, however there were no significant differences in propofol dosing to achieve goal RASS in mcg/kg/min or in mg/min. The higher number of propofol boluses utilized in the IBW may have been a result of targeting relatively deeper RASS goal at baseline in this group.
IBW dosing did not take more time or cause excessive use or doses of concomitant sedatives or agents for agitation to achieve target RASS. Additionally, using IBW led to similar requirements of vasoactive agents, incidence of bradycardia, or hypertriglyceridemia. There were no significant differences between groups in propofol dose to achieve target RASS, duration of mechanical ventilation, duration of propofol, or re-intubations. There was a longer time from discontinuation of propofol to extubation in the IBW group, showing that a switch to another sedative may have been used prior to extubation. No significant differences in ICU or hospital length of stay or mortality were found. Overall, there were no apparent benefits nor disadvantages in using IBW vs. ABW dosing of propofol with respect to the efficacy and safety outcomes analyzed.
Our population had a significant difference in target RASS goal for the propofol infusion order; thus, a subgroup analysis of patients targeting light sedation (RASS 0 to −2) of 34 patients was conducted finding no difference in the primary outcome of time to goal RASS between groups, though the IBW group may have spent less time in goal RASS. This matched cohort also had no difference in up or down titrations between groups, though we see fewer down titrations required in the IBW group. This finding suggests we may have expected less titrations and lower doses to achieve goal RASS in the IBW group if the target RASS goals were similar between groups.
This multi-centered study is the first head-to-head study comparing IBW to ABW with clinically relevant endpoints that accounted for confounders such as adjunct sedatives and anti-psychotics. A major limitation of the study is the significant difference in target RASS goals between groups where the IBW group targeted a relatively deeper initial sedation goal. The significant difference between groups with initial target RASS goal for propofol dosing may have confounded the outcomes. Therefore, a subgroup analysis was performed for patients reaching target RASS (n=36) and in patients targeting light sedation (n=34) which limited our study sample size even further. To monitor pharmacodynamic difference in our study sample, data analysis stratified by non-obese (BMI <30 kg/m2) vs. obese (BMI ≥30 kg/m2) was reported (Table 8), though each subgroup sample is small. As a retrospective chart review, the data is limited by the presence and accuracy of documentation of target RASS goals by physicians and RASS scores by nurses. Future studies with a larger sample size and matched cohorts based on initial goal RASS would be required to detect a difference in outcomes.
Table 8
| Outcome | IBW | ABW | |||
|---|---|---|---|---|---|
| Non-obese (n=15) | Obese (n=13) | Non-obese (n=10) | Obese (n=12) | ||
| Time to goal RASS | 4 [2–6] | 6 [2–10] | 3 [1–6] | 4 [1–5] | |
| Fentanyl infusion, n [%] | 6 [40] | 5 [50] | 6 [46] | 5 [42] | |
| Infusion rate, mcg/h | 50 [31–88] | 125 [50–150] | 100 [75–150] | 50 [44–69] | |
| Dexmedetomidine Infusion, n [%] | 2 [13] | 2 [20] | 1 [8] | – | |
| Infusion rate, mcg/kg/h | 1.25 [1–1] | 0.95 [1–1] | 2 [2–2] | – | |
| Propofol bolus, n [%] | 5 [33] | 2 [20] | 1 [8] | 1 [8] | |
| Propofol bolus dose, mcg | 40 [20–50] | 75 [63–88] | 20 [20–20] | 50 [50–50] | |
| Propofol dose to achieve goal RASS, mcg/kg/min | 32.5 [23–44] | 30 [8–36] | 40 [30–44] | 10 [8–36] | |
| Propofol dose to achieve goal RASS, mg/min | 2.0855 [2–3] | 1.5 [1–2] | 2.772 [2–4] | 1.0295 [1–3] | |
| Time from discontinuation of propofol to extubation, hours | 24.3 [17–113] | 30.2 [11–100] | 72.3 [23–8,783] | 255.1 [68–8,789] | |
| Duration of mechanical ventilation, days | 6 [2–9] | 5.5 [3–14] | 5 [3–8] | 6.5 [3–9] | |
| Duration of propofol, hours | 39 [28–76] | 50 [35–108] | 73 [50–85] | 50.5 [46–73] | |
| Re-intubations, n [%] | – | 2 [20] | 3 [23] | 3 [25] | |
| ICU LOS, days | 6 [3–14] | 8.5 [4–13] | 12 [8–21] | 9.5 [8–13] | |
| Hospital LOS, days | 15 [9–26] | 21 [12–35] | 21 [15–27] | 13 [9–24] | |
| Mortality | 3 [20] | 2 [20] | 2 [15] | 4 [33] | |
All values are expressed as median [IQR] unless otherwise noted. BMI, body mass index; IBW, ideal body weight; ABW, actual body weight; RASS, Richmond Agitation Sedation Scale; ICU, intensive care unit; LOS, length of stay; IQR, interquartile range.
Conclusions
In our study, the time to target RASS was similar between IBW-based propofol dosing and ABW-based propofol dosing. The subgroup analysis of patients targeting light sedation also showed no difference in time to target RASS. Though rare, adverse effects occurred at a similar rate between groups and there were no differences detected in adjunct sedative or vasopressor requirements. IBW-based dosing may be a potential alternative dosing strategy of continuous propofol infusion with similar efficacy and safety profile to ABW-dosing. Larger studies with matched cohorts are required to confirm these findings and make a definite conclusion.
Acknowledgments
Abstract was adapted with permission from Wolters Kluwer Health, Inc.: Gimelbrand, Ilana; Merchan, Cristian; Bhatt, Prachi; Wassner, Chanie; Altshuler, Diana; Adelman, Mark; Elnadoury, Ola. 917: Efficacy and Safety of Propofol Dosing Based on Ideal Body Weight versus Actual Body Weight. Critical Care Medicine: January 2022 - Volume 50 - Issue 1 - p 455. doi: 10.1097/01.ccm.0000809992.85603.03.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-21-115/rc
Data Sharing Statement: Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-21-115/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-21-115/coif). OE reports stock options from Regeneron Pharmaceuticals, Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by New York University (NYU) Langone Health Institutional Review Board (No. s20-02067) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Diprivan® (propofol) Injectable Emulsion [package insert]. Lake Zurich, Illinois: Fresenius Kabi USA, LLC.; 2014.
- Grasshoff C, Gillessen T. Effects of propofol on N-methyl-D-aspartate receptor-mediated calcium increase in cultured rat cerebrocortical neurons. Eur J Anaesthesiol 2005;22:467-70. [Crossref] [PubMed]
- Kotani Y, Shimazawa M, Yoshimura S, et al. The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther 2008;14:95-106. [Crossref] [PubMed]
- Devlin JW, Skrobik Y, Gélinas C, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med 2018;46:e825-e873. [Crossref] [PubMed]
- Erstad BL, Barletta JF. Drug dosing in the critically ill obese patient-a focus on sedation, analgesia, and delirium. Crit Care 2020;24:315. [Crossref] [PubMed]
- Ingrande J, Brodsky JB, Lemmens HJ. Lean body weight scalar for the anesthetic induction dose of propofol in morbidly obese subjects. Anesth Analg 2011;113:57-62. [Crossref] [PubMed]
- Tsaousi G, Fyntanidou B, Stavrou G, et al. Propofol Sedation for Intragastric Balloon Removal: Looking for the Optimal Body Weight Descriptor. Obes Surg 2019;29:3882-90. [Crossref] [PubMed]
- Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia 2007;62:690-701. [Crossref] [PubMed]
- Araújo AM, Machado HS, Falcão AC, et al. Reliability of body-weight scalars on the assessment of propofol induction dose in obese patients. Acta Anaesthesiol Scand 2018;62:464-73. [Crossref] [PubMed]
- Johnson AL, Altshuler D, Schwartz DR, et al. Effect of obesity on propofol dosing requirements in mechanically ventilated patients in a medical intensive care unit. J Emerg Crit Care Med 2018;2:97. [Crossref]
- Chassard D, Berrada K, Bryssine B, et al. Influence of body compartments on propofol induction dose in female patients. Acta Anaesthesiol Scand 1996;40:889-91. [Crossref] [PubMed]
- McLeay SC, Morrish GA, Kirkpatrick CM, et al. The relationship between drug clearance and body size: systematic review and meta-analysis of the literature published from 2000 to 2007. Clin Pharmacokinet 2012;51:319-30. [Crossref] [PubMed]
Cite this article as: Gimelbrand I, Merchan C, Bhatt P, Wassner C, Altshuler D, Adelman MH, Elnadoury O. Efficacy and safety of propofol dosing based on ideal body weight vs. actual body weight: a retrospective cohort study. J Emerg Crit Care Med 2022;6:20.


