
Organophosphates pesticide poisoning: a peculiar case report
Introduction
Organophosphates compounds (OPs) are synthetized to produce pesticides in agricultural industry since the 1930s; they are also used in medicine for treatment of diseases such as glaucoma, Alzheimer’s or myasthenia gravis and, to revers of neuromuscular block in anesthesia. Unfortunately, the potential use of OPs as nerve agents in terrorist attacks should also be reported (1).
In developing countries, due to poverty, unemployment and social degradation, these products are often taken for self-harm. Gunnell et al. report approximately 30% of suicide cases globally to be caused by OP intoxication (2). It is estimated that around 3,000,000 per people worldwide are exposed to OPs, with up to 300,000 deaths (3).
Generally, OPs toxicity was caused by accidental, intentional ingestion or exposure to agricultural pesticides (3). However, other potential causes of OPs toxicity include ingestion of contaminated food or contact with contaminated clothing. OPs intoxication can occur in different ways: ingestion, inhalation, and contact with skin, eyes, and mucous membranes. The diagnosis is clinical and based on anamnestic data, exposure to OPs for self-injurious or accidental, provided by victims or people informed of the facts (4).
Mortality from OPs intoxication ranges from 3% to 30% and often correlates with delayed diagnosis or improper treatment (5). This rises to 50% for those with prolonged mechanical ventilation, complications such as pneumonia or weaning difficulties (6). In 2/3 of the most severe cases, the pesticides involved are parathion and dimethoate (3).
The OPs bind cholinesterase enzymes responsible for the hydrolysis of acetylcholine (ACh), leading to their inactivation. Failure to metabolize ACh determines its accumulation at the synaptic level, initially causing over-stimulation of nervous system (NS) and, subsequently, complete block in nerve impulses transmission (7).
Clinical manifestations observed in OPs agents were due to the action exerted by the latter on muscarinic (M) and nicotinic (N) receptors (8). Muscarinic syndrome (acute cholinergic syndrome): early presentation, characterized by miosis, impaired consciousness, hyper-salivation, agitation, bradycardia (sometimes tachycardia), muscle weakness, vomiting and diarrhea (9). Nicotinic syndrome or intermediate syndrome (IMS): defined in this way as it usually follows muscarinic syndrome and precedes delayed neuropathy. It occurs 24–96 h after exposure to OP, with ascending paralysis and death due to the involvement of the respiratory muscles (6). Delayed neuropathy: 2/3 weeks after poisoning, with extremities weakness and, sometimes, respiratory failure due to involvement of phrenic nerve (7).
In Italy, the use of dimethoate has undergone significant restrictions. Due to the high toxicity, a European directive (EU regulation No. 2019/1090), revoked its use from 01/10/2019. However, due to pressure from the agri-food sector, with a decree of 06/2020 the companies were authorized to re-market products based on dimethoate from 1/07/2020 and for a period of 120 days [Article 53, paragraph 1, Regulation (EC) No. 1107/2009].
We present a rare case of OPs intoxication occurred in developed European country, where timely resuscitation treatment with ventilatory/hemodynamic support and treatment with anticholinesterases led to a favorable outcome. We present the following case in accordance with the CARE reporting checklist (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-22-64/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
A male, 84 years old, was find in a state of unconsciousness in his agricultural field and transported in red code to the Emergency Department (ED) of Mazzini Hospital in Teramo.
Upon arrival, no anamnestic data were available. Neurological status with Glasgow Coma Scale (GCS) of 3, muscle and peribuccal fasciculation, myotic pupils, hyper-lacrimation, skin face hyperemia, diaphoresis, urinary incontinence, and respiratory failure with stertorous breathing and bronchoconstriction due to bronchorrhea were observed. Due to hemodynamic instability and respiratory failure orotracheal intubation was performed, and volemic resuscitation was stared.
On the electrocardiogram (ECG), atrial flutter (AF) was detected (Figure 1A: patient’s previous normal ECG; Figure 1B: AF), electrical cardioversion (ECV) attempt was ineffective.
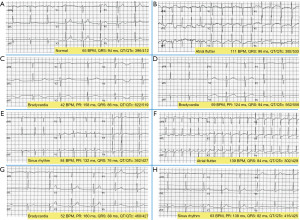
It was also performed a computed tomography (CT) scan brain with contrast that excluded intracranial acuities. Laboratory data and vital parameters at ED arrivals are summarize in Table 1.
Table 1
| Variables | Value | Range |
|---|---|---|
| Laboratory variables | ||
| Myoglobin (ng/mL) | 1,019 | 28–72 |
| CK-MB (mg/dL) | 9.4 | 0.0–3.6 |
| Troponin (pg/mL) | 70.1 | 14–50 |
| Serum creatinine (mg/dL) | 2.1 | 0.7–1.2 |
| BUN (mg/dL) | 58 | 10–50 |
| PLT (×103/µL) | 74* | 150–450 |
| Leucocyte (×103/µL) | 13.83 | 4–10 |
| Lymphocyte (×103/µL) | 1.8 | 1.00–4.00 |
| Neutrophil (×103/µL) | 10.40 | 2.00–7.00 |
| Glycaemia (mg/dL) | 287 | 60–125 |
| Lactate (mg/dL) | 50 | <18 |
| Pseudocholinesterase (U/L) | 7.836 | 5.320–12.920 |
| ED vital signs | ||
| APACHE 2 | 29 | 0–71 |
| SAPS II | 71 | 0–163 |
| GCS | 3 | 3–15 |
| SBP (mmHg) | 80 | 120–155 |
| DBP (mmHg) | 50 | 65–90 |
| HR (BPM) | >140 AF | 60–100 |
| Haemoglobin (g/dL) | 14 | 14–18 |
| O2 saturation % | 85 | 95–100 |
*, PLT decreased at 6 h from ED admission, entry count was 234 (×103/µL). AF, atrial flutter; BUN, blood urea nitrogen; CK-MB, creatine kinase-MB; PLT, platelet; ED, emergency department; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; BPM, beats per minute; GCS, Glasgow Coma Scale; APACHE, acute physiologic assessment and chronic health evaluation; SAPS; simplified acute physiology score.
After contacting the family members, it was clear that the subject, upon discovery, was in the countryside intent on administering dimethoate-based organophosphorus pesticide on olive plants.
Atropine was immediately initiated (0.5 mg iv, every 15–20 min) and the national reference Poison Control Center of Pavia was contacted. After transfer to the intensive care unit (ICU), we proceeded as indicated: accurate cleansing decontamination of the skin; execution of esophagogastroduodenoscopy (EGDS) for excluding poisoning by the digestive route; administration of antidote pralidoxime, 1 g iv bolus, followed by continuous infusion (500 mg/h for 48 h; then 250 mg/h for 12 h, finally 125 mg/h for further 12 h).
During ICU stay, several episodes of respiratory or circulation failure were treated. Twenty-four h after OPs intoxication, extreme bradycardia with QT lengthening (Figure 1C,1D), unresponsive to atropine, with the need for external pacing and intravenous (IV) dobutamine. Fasciculation in the lower limbs, abundant bronchial secretions was observed and treated. Subsequently, there was a slow and progressive clinical, respiratory and cardiocirculatory improvement, with restoration of sinus rhythm (Figure 1E), until extubation occurred on the 10th day.
At day 13th onset of psychomotor agitation, cognitive deterioration, reduction of ventilatory performance up to respiratory arrest with need for re-intubation were present. Marked hypotension with AF undergoing ECV 200 J was observed. At day 21 cardiocirculatory arrest (CCA) with asystole occurred and return of spontaneous circulation (ROSC) after cardiopulmonary resuscitation (CPR) and adrenaline 1 mg iv was described. Percutaneous tracheostomy was performed on day 23 and subsequent AF with 1st degree A-V block (atrio-ventricular block), ventricular extra systoles, was treated with amiodarone (Figure 1F). From day 25, after clinical picture normalization suspension of sedation and weaning was perform. Patient was alert, cooperative, without neurological deficits. Hemodynamics were stable and there were no significant electrocardiographic changes except mild bradycardia with QT interval within limits (Figure 1G). Therefore, he was transferred to a rehabilitation institution.
A 2-year follow-up, subject was finding in excellent general condition, without any neurological deficit. Also, ECG was normal (Figure 1H). Amnesia, regarding the incident and the way of intoxication (inhalation, contact with skin and/or mucous membranes), was reported.
Discussion
Our case was characterized by succession of all the clinical manifestation that can occur in OP poisoning intoxication. Timely and prolonged resuscitation treatment not only guaranteed the patient’s survival, but also allowed various pathophysiological mechanisms that characterize the intoxications of cholinesterase inhibitors to emerge.
Upon finding a compromised neurological status, agonal breathing and hemodynamic instability, the airways were secured by intubation and both respiratory and circulatory functions were supported with IMV, fluids and amines. Specifically, in case of OP poisoning, lower GCS have been considered predictors for high incidence of respiratory complications and mortality (5).
The presence of clinical signs suggestive of muscarinic syndrome (8-10), associated with reported exposure to OPs, allowed to quickly start administration of drugs considered able to counteract OPs action on cholinergic receptors such as atropine and pralidoxime (11).
Atropine is a known ACh antagonist for M receptors. Practiced for a long time, from arrival in the ED and up to clinical normalization. Continuous infusion was not choosing as cholinergic symptoms are often accompanied by hyperkinetic arrhythmias, requiring beta-blockers or antiarrhythmic to control heart rate. Atropine, essential for secretion control, was administered cautiously, with repeated boluses of 0.5 mg (11).
Cholinesterase reactivates spontaneously, but slowly. Pharmacological agents such as oximes that act through hydrolysis of the enzyme-OP compounds before the bond becomes irreversible can favor the process (11).
Although there is no broad consensus on oximes efficacy (pralidoxime), they are recommended by the World Health Organization (WHO) Guidelines in cases of OPs intoxication (12).
Despite drug therapy, the patient required prolonged mechanic ventilation. This is most likely attributable to the onset of the so-called IMS due to the blocking of the nicotinic receptors for ACh, which occurs when 80% of cholinesterases are inactivated. Typically, onset 24–96 h after exposure to the OPs, causes fasciculation and proximal muscle weakness, with ascending paralysis leading to respiratory failure, the main cause of death (9,13).
The literature describes a form of delayed polyneuropathy, which is observed between 2 and 4 weeks after exposure to OP and especially in relation to agents capable of overcoming the blood brain barrier such as parathion and dimethoate (13).
The binding of the OPs to an esterase called neuropathy target esterase (NTE), abundant at the neuronal level, seems to cause inactivation of the enzyme itself with consequent accumulation of phosphatidylcholine in the brain and neurodegeneration. This neuropathy, which often affects the extremities and, sometimes, the phrenic nerve, could explain respiratory arrest and neurological deficits found (9,13).
Numerous arrhythmias occurred (Figure 1B-1D,1F). Conduction disturbances and electrocardiographic abnormalities are extensively described in OPs intoxication (14).
The mechanism is not clear but since the cholinergic innervation of the heart is both chronotropic and negative inotropic, this would result in myocardial conduction slowdown and ventricular repolarization delay. The lack of serum troponin elevation, however, would seem to exclude direct myocardial injury.
Furthermore, QT tract elongation found, would confirm the ability of the OPs to determine alterations in cardiac conduction. Some studies have correlated this aspect to severity and it is considered a negative prognostic factor, particularly in dimethoate poisoning (14,15).
To confirm what has been stated, the ECGs performed previously (Figure 1A) and during the 3-month follow-up did not show QT alterations (Figure 1H).
In our case, there was severe and persistent hypotension, refractory to fluid therapy, conditioning distributive shock and the need for therapy with vasopressors. Animal studies have shown that in acute dimethoate poisoning, multiple mechanisms are involved: Ach action on M receptors present on vascular endothelium with associated oxidative damage; inhibition of baroreceptor reflexes by effect on N receptors; histamine-induced hypotension due to mast cell activation (16).
Hyperglycemia is frequently found in critically ill patients and, as is known, has a negative impact on the outcome (17).
Due to activation of sympathetic NS, high glycemic values in OPs poisoning are correlated with severity of intoxication and are considered predictors for the development of IMS (17).
Although, patient was suffering from type II diabetes, during the critical phase, glycemic control was difficult, despite IV insulin therapy and execution of additional boluses. Conversely, gradual improvement has seen a normalization of blood glucose levels.
Lactatemia and serum creatinine increase found probably have multifactorial genesis. They are likely related to both muscle damage caused by fasciculations (expressed with increased levels of myoglobin) and organophosphorus-induced acute tubular damage, resulting in hypokalemia (8).
Serum cholinesterases showed a biphasic trend in acute decrease and normalize in healing. However, it was not possible to measure them in our center. In contrast, pseudocholinesterases resulted in range. This depends on high enzymes polymorphism, with different sensitivity in relation to OPs type. In dimethoate poisoning they often remain normal and therefore are not useful in the diagnostic phase or in assessing the progress of the disease.
Hematological changes such as leukocytosis with neutropenia and thrombocytopenia, have been frequently reported. It is not known whether OPs can acutely induce damage to the synthesis of the megakaryocytic line, while it seems that the effects on the white blood cells are due to a physiological response to stress. Conversely, neutropenia seems to be related to cellular margination rather than to an effective numerical elements reduction (18).
In case of OPs intoxication, rapid recognition of signs and symptoms together with the responsible agent identification are essential for setting up drug treatment with antagonists/antidotes. However, as emerged from our case and in line with the literature (19), resuscitation maneuvers performed upon arrival and continued until clinical resolution (mechanical ventilation and support of the circulation with fluids and amines) played a central role in determining a favorable outcome. However, it should be emphasized that pralidoxime administration, even if early, did not lead to a rapid resolution of the clinical picture nor did it prevent the onset of IMS or later neurological complications. Moreover, the effectiveness of the drug is still controversial, especially in dimethoate poisoning.
On the contrary, atropine was found to be effective above all in secretions control, although in the early phase its use was limited by the presence of tachycardia and hypotension, despite the administration of beta-blockers. Furthermore, at 48 h the bradycardia pushed with QT lengthening was not responsive to atropine and required an external pacing placement. This has led us to think that the effects on the cardiovascular system in OPs intoxication are extremely varied and unpredictable. Relying on antidotes can certainly be helpful, but of primary importance are resuscitation maneuvers and the vital functions support. The normalization of the electrocardiographic picture, hemodynamic stabilization, lactates, and glycemic control suggest that trend of these parameters may be related to favorable clinical evolution and therefore have prognostic value (19).
There were no laboratory tests useful in the diagnostic phase, serum cholinesterase can serve as confirmation of toxic exposure, and their dosage was not a routine test, requires a long time. Conversely, pseudocholinesterases were not sensitive or specific in dimethoate intoxication (20).
This leads us to underline that at an early-stage anamnestic data collection can be of vital importance to implement adequate resuscitation measures.
Sometimes, however, especially when exposure is accidental as in our case, it is not always possible to understand how intoxication occurred. We believe that our there were a multimodal exposure. The use of spray tools in the absence of adequate personal protective equipment (PPE) may have resulted in both inhalation and contact with mucous membranes. Although dimethoate is not easily absorbed through the skin, vasodilation linked to high temperatures, which occur in the height of summer in the Italian countryside, could have favored dermis reabsorption, to the face and other uncovered areas. Hyperemia showed in ED admission localized on cranial regions and erythematous areas in chest and abdomen, were also indicative of inflamed skin, with altered barrier mechanisms and associated vasoplegia. As recently reported, mortality in elderly patients with OP intoxication was 19.7%, with the development of cholinergic syndrome by 100%, respiratory failure in 52.1% of cases, IMS (15.5%) aspiration pneumonia (50.7%), acute kidney injury (43.7%), severe consciousness disturbance (25.4%), shock (14.1%), seizures (4.2%), and only delayed neuropathy (4.2%) (21).
Shock appears to represent a significant risk for mortality in these elderly patients. Prompt diagnosis of OPs intoxication, fluid resuscitation followed by infusion of inotropic drugs, and prompts administration of antidotes such as anticholinergic drugs and oxime, appear to be crucial for the course of the disease and prevent fatal consequences (21).
Our case shows the entire clinical diagnostic and therapeutic path of acute OPs intoxication in an elderly patient of a developed European country such as Italy, where, given the low frequency of such poisonings, there is a challenging in differential diagnosis in the ED. The onset characterized by symptomatologic severity with the simultaneous dramatic involvement of the cardiovascular-respiratory and NS, advanced age, associated comorbidities, careful hemodynamic and respiratory monitoring with complete electrocardiographic documentation, the multimodal exposure, and finally the excellent clinical outcomes, makes our case of particular interest. In fact, OPs intoxications are reported due to ingestion with initially mild clinical presentation or involving only one apparatus and which subsequently over a few hours worsen up to cardiorespiratory arrest or coma. In our case, since the clinical presentation in ED could mimic several different pathological conditions (trauma, sepsis, stroke, seizure, hypoglycemia) that require careful differential diagnosis and rapid supportive therapy of vital signs, it was essential to collect a good medical history to orientate towards organophosphorus poisoning.
Furthermore, the follow-up performed 2 years after discharge from intensive care shows how aggressive intensive treatment can affect long-term complications (delayed neuropathy) in organophosphorus intoxication survivors or in any case in mitigating or slowing them down. Furthermore, such detailed and complete follow-up periods are rarely reported in elderly patient.
Conclusions
In conclusion, the absorption of a non-lethal dose certainly avoided untimely death. A timely resuscitation treatment and the application of intensive care for a prolonged time, with concomitant treatment of all sequential complications, and antidote administration, made it possible to guarantee a favorable outcome, with recovery and reduction of late sequelae; and no delayed neuropathy as verified at 3th, 12th, and 24th months checks.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-22-64/rc
Peer Review File: Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-22-64/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-22-64/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jett DA, Spriggs SM. Translational research on chemical nerve agents. Neurobiol Dis 2020;133:104335. [Crossref] [PubMed]
- Gunnell D, Eddleston M, Phillips MR, et al. The global distribution of fatal pesticide self-poisoning: systematic review. BMC Public Health 2007;7:357. [Crossref] [PubMed]
- Karunarathne A, Gunnell D, Konradsen F, et al. How many premature deaths from pesticide suicide have occurred since the agricultural Green Revolution? Clin Toxicol (Phila) 2020;58:227-32. [Crossref] [PubMed]
- Wu ML, Deng JF, Tsai WJ, et al. Food poisoning due to methamidophos-contaminated vegetables. J Toxicol Clin Toxicol 2001;39:333-6. [Crossref] [PubMed]
- Davies JO, Eddleston M, Buckley NA. Predicting outcome in acute organophosphorus poisoning with a poison severity score or the Glasgow coma scale. QJM 2008;101:371-9. [Crossref] [PubMed]
- Çolak Ş, Erdoğan MÖ, Baydin A, et al. Epidemiology of organophosphate intoxication and predictors of intermediate syndrome. Turk J Med Sci 2014;44:279-82. [Crossref] [PubMed]
- Pannu AK, Bhalla A, Vishnu RI, et al. Organophosphate induced delayed neuropathy after an acute cholinergic crisis in self-poisoning. Clin Toxicol (Phila) 2021;59:488-92. [Crossref] [PubMed]
- Singh G, Khurana D. Neurology of acute organophosphate poisoning. Neurol India 2009;57:119-25. [Crossref] [PubMed]
- Dawson AH, Eddleston M, Senarathna L, et al. Acute human lethal toxicity of agricultural pesticides: a prospective cohort study. PLoS Med 2010;7:e1000357. [Crossref] [PubMed]
- Hulse EJ, Davies JO, Simpson AJ, et al. Respiratory complications of organophosphorus nerve agent and insecticide poisoning. Implications for respiratory and critical care. Am J Respir Crit Care Med 2014;190:1342-54. Erratum in: Am J Respir Crit Care Med 2019 Oct 1;200(7):946-948. [Crossref] [PubMed]
- Eddleston M. Novel Clinical Toxicology and Pharmacology of Organophosphorus Insecticide Self-Poisoning. Annu Rev Pharmacol Toxicol 2019;59:341-60. [Crossref] [PubMed]
- Syed S, Gurcoo SA, Farooqui AK, et al. Is the World Health Organization-recommended dose of pralidoxime effective in the treatment of organophosphorus poisoning? A randomized, double-blinded and placebo-controlled trial. Saudi J Anaesth 2015;9:49-54. [Crossref] [PubMed]
- Giyanwani PR, Zubair U, Salam O, et al. Respiratory Failure Following Organophosphate Poisoning: A Literature Review. Cureus 2017;9:e1651. [Crossref] [PubMed]
- Shadnia S, Okazi A, Akhlaghi N, et al. Prognostic value of long QT interval in acute and severe organophosphate poisoning. J Med Toxicol 2009;5:196-9. [Crossref] [PubMed]
- Grmec S, Mally S, Klemen P. Glasgow Coma Scale score and QTc interval in the prognosis of organophosphate poisoning. Acad Emerg Med 2004;11:925-30. [Crossref] [PubMed]
- Yavuz T, Delibas N, Yildirim B, et al. Vascular wall damage in rats induced by organophosphorus insecticide methidathion. Toxicol Lett 2005;155:59-64. [Crossref] [PubMed]
- Xiao X, Clark JM, Park Y. Potential contribution of insecticide exposure and development of obesity and type 2 diabetes. Food Chem Toxicol 2017;105:456-74. [Crossref] [PubMed]
- Mu Y, Hu B, Gao N, et al. Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning. Open Life Sci 2021;16:703-10. [Crossref] [PubMed]
- Sungur M, Güven M. Intensive care management of organophosphate insecticide poisoning. Crit Care 2001;5:211-5. [Crossref] [PubMed]
- Aygun D, Erenler AK, Karatas AD, et al. Intermediate syndrome following acute organophosphate poisoning: correlation with initial serum levels of muscle enzymes. Basic Clin Pharmacol Toxicol 2007;100:201-4. [Crossref] [PubMed]
- Yu JR, Hou YC, Fu JF, et al. Outcomes of elderly patients with organophosphate intoxication. Sci Rep 2021;11:11615. [Crossref] [PubMed]
Cite this article as: Faluomi M, Cialini M, Naviganti M, Mastromauro A, Marinangeli F, Angeletti C. Organophosphates pesticide poisoning: a peculiar case report. J Emerg Crit Care Med 2022;6:30.


