
Treatment of respiratory syncytial virus with palivizumab in an adult liver transplant recipient: a case report
Highlight box
Key findings
• Utilization of low dose palivizumab, in conjunction with standard of care, resulted in improvement of symptoms as well as radiologic findings in an adult liver transplant patient with respiratory syncytial virus (RSV).
What is known and what is new?
• Palivizumab is used in infants for prophylaxis as well as treatment of RSV, and limited data is available that supports the use of palivizumab in adult immunocompromised patients who have undergone solid organ transplant.
• This case report describes the first reported use of palivizumab in an adult liver transplant recipient with RSV.
What is the implication, and what should change now?
• Palivizumab may serve as a viable option for adult immunocompromised patients who have undergone solid organ transplant that have not adequately responded to standard of care. Additional randomized trials are needed to further determine the utility of palivizumab in this patient population.
Introduction
Respiratory syncytial virus (RSV) is an RNA virus of the paramyxoviridae family which causes upper and lower respiratory tract infections (URTIs and LRTIs) (1). RSV is particularly dangerous for infants and young children; it is the most common cause of LRTI and is associated with a high mortality burden (2). Infection with RSV is mostly restricted to superficial respiratory epithelium cells and can affect cells from the trachea, bronchi, and bronchioles. The heterogeneity of RSV results in its varying disease burden from mild disease in most adults to severe pneumonia in infants, immunocompromised patients, or the institutionalized elderly (3).
Despite a near universal history of infection during infancy, adult patients may become re-infected with RSV repeatedly throughout their lives. The mortality rate in adults over 50 years of age is 6–8% (2). Immunocompromised adults, such as those with solid organ transplantation or hematopoietic stem cell transplantation, are at particular risk, with infection rates varying from 5–50% (1). Treatment for RSV in adult patient populations is generally limited to supportive care (4). However, some success has been reported in treating immunocompromised adult patients with palivizumab (5-9), a humanized monoclonal antibody against RSV which is currently approved for prevention of RSV in infants and children under the age of 2 years (10). We report the first known case of RSV treated with palivizumab in an immunocompromised adult patient with a history of liver transplant in accordance with the CARE reporting checklist (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-22-59/rc).
Case presentation
A 50-year-old female who underwent orthotopic liver transplant secondary to non-alcoholic steatohepatitis approximately 7 years prior to admission, presented to the hospital with shortness of breath, cough, and acute hypoxemic respiratory failure concerning for multifocal pneumonia. Pertinent social history includes an active 26 pack-year smoking history.
Initial diagnostic data were collected on day 1 of admission. A nasopharyngeal sample was tested for COVID-19 via polymerase chain reaction (PCR) and was found to be negative. Chest computed tomography and X-ray were notable for bilateral ground-glass opacities and negative for pulmonary embolism. Sputum and blood cultures were collected and empiric antibiotics were initiated pending results of culture data. Her initial oxygen saturation was 83% on room air which improved to 94–95% on nasal cannula at 5 L/min. Her home regimen for immunosuppression with tacrolimus and mycophenolate mofetil (MMF) was continued. At presentation, her white blood cell count was 4.6×109 cells/L with 35.3% lymphocytes and her C-reactive protein was 21.95 mg/dL.
On day 2 of admission, the BioFire® Respiratory 2.1 Panel was positive for RSV in a nasopharyngeal swab and she had a negative methicillin-resistant Staphylococcus aureus nasal swab, so vancomycin was discontinued. MMF was also held. On day 3, she experienced increased work of breathing and desaturation to 50%. She was placed on a non-rebreather mask at 15 L/min and a follow-up arterial blood gas showed acute hypercapnic respiratory failure with associated respiratory acidosis (pH 7.15, pCO2 79 mmHg, pO2 234 mmHg, bicarbonate 27.6 mmol/L, SaO2 100%). She was emergently intubated and transferred to the intensive care unit. At this time, ceftriaxone was escalated to piperacillin/tazobactam to empirically cover multifocal pneumonia and RSV treatment was initiated with ribavirin oral suspension 400 mg twice daily and hydrocortisone 100 mg intravenous (IV) once followed by methylprednisolone 40 mg IV daily. Serum immunoglobulin was 1,096 mg/dL so no treatment with intravenous immunoglobulin (IVIG) was indicated.
Antibiotics were discontinued on day 5 and up until day 6, she remained intubated and on methylprednisolone and ribavirin. Despite antiviral treatment and supportive care with IV steroids, chest imaging was showing worsening pulmonary infiltrates and congestion. Progression can be seen in Figure 1A,1B which depict chest X-rays from day 3 following intubation to day 6. Due to the patient’s lack of improvement on these therapies, it was determined that she might benefit from off-label treatment with palivizumab 700 mg (approximately 11 mg/kg, using ideal body weight) intramuscularly (administered as 4 injections) once.
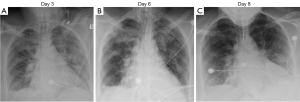
On day 8 her chest imaging had improved (Figure 1C) and she was extubated to high flow nasal cannula (60 L/min with 40% FiO2); following extubation her arterial blood gas showed pH 7.45, pCO2 47 mmHg, pO2 104 mmHg, bicarbonate 32.1 mmol/L, SaO2 99%. On day 9, she was transitioned to room air and transferred out of the intensive care unit (ICU). Ribavirin was continued for a total of 10 days and she was discharged home on day 13 with a short course taper of prednisone.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
This case represents the first known publication of the use of palivizumab in an adult liver transplant recipient. The treatment of severe RSV infections in adults is limited by the lack of available treatment options and supportive care is the mainstay of therapy (4). However, given that the morbidity and mortality associated with RSV infections can be significant, it’s essential to understand its diagnosis and the alternative treatment options available (1,2).
In our case, RSV infection was detected utilizing the BioFire® RP2.1 Panel which is a multiplex PCR assay (11). This diagnostic assay offers the advantage of testing for multiple viral and bacterial respiratory infections in a single panel; however, its turn-around-time is longer than available rapid-antigen tests at 45 minutes. Furthermore, PCR assays are more sensitive than antigen-based tests, particularly in adult patients (11,12). Therefore, particularly in adult immunocompromised patients, the most sensitive and specific assay available should be utilized to detect RSV, along with other viral, bacterial, and fungal tests in order to differentiate between infections with similar presentations
Ribavirin is a synthetic guanosine nucleoside analog with anti-viral activity which has been successfully used for the treatment of RSV respiratory tract infections. When used in combination with palivizumab, it is often administered via the inhalation route (6-9). A large systematic review of the use of aerosolized ribavirin for treatment of RSV in adult immunocompromised patients found a mortality benefit in 4 out of 15 cited articles when treatment was initiated early, prior to mechanical ventilation. Adverse events reported included psychiatric manifestations (anxiety, depression, and a feeling of isolation) and wheezing/bronchospasm (13). Aside from the adverse events, a major limitation of the administration of ribavirin via the inhalation route is its cost. While not approved by the Food and Drug Administration for the treatment of RSV, oral formulations of ribavirin have been used off-label for this purpose (10). Multiple studies have compared oral and inhaled ribavirin and found similar outcomes associated with lung function and mortality (14-16). Furthermore, the oral formulation has been associated with significant cost savings (15,16). Despite its reported success in the treatment of RSV, ribavirin therapy is still associated with a 30-day mortality rate of up to 26% (13,17). Therefore, the utilization of additional agents may be beneficial in selecting high-risk patients in order to attempt to reduce this risk.
Palivizumab is a humanized monoclonal antibody which binds to and prevents the entry of RSV into human cells. However, in the decades since initial approval, palivizumab has been used in adult patients for treatment of RSV, particularly in the immunocompromised population. Table 1 presents a summary of the previous use of palivizumab in adult patients for treatment of RSV. This includes data in solid organ transplant (SOT) recipients and in patients with hematologic malignancies or hematopoietic stem cell transplant (HSCT). Several studies demonstrated success with treatment (5-9). Mortality in the majority of these cases was lower when compared to treatment with ribavirin alone, with only one small study having comparable 30-day mortality (7). The majority of these studies utilized a dosing regimen of 15 mg/kg IV once (6-8); however, success has also been noted with lower doses of 7.5 or 8 mg/kg (5,9). Notably, the largest study of palivizumab in immunocompromised patients found no difference in progression of disease from URTI to LRTI or in 1-year overall survival (18). Collectively, existing literature suggests that palivizumab may be a reasonable therapy to administer in immunocompromised patients. However, cost is also an important consideration, with a single 100 mg vial costing $3,698.58 (average wholesale price) (19). In infants, its use is considered cost-effective for the prevention of RSV; however, further data would be necessary to determine this factor in adult patients when used as treatment (20). Current guidelines for the treatment of respiratory viral infections in SOT recipients do not recommend one treatment regimen over another (12). However, they do state that treatment with a combination of ribavirin, steroids, IVIG, and/or palivizumab is preferred over supportive care alone (12).
Table 1
| Reference | Report type | Population | Age (years) | Treatment | Outcomes |
|---|---|---|---|---|---|
| Banna et al. (5) | Case report | HSCT patient | 56 | Betamethasone 4 mg IV twice daily; palivizumab 8 mg/kg once | Complete resolution of fever the day following palivizumab; oxygen therapy was discontinued 10 days after therapy |
| Liu et al. (6) | Retrospective review | Adult lung or heart-lung transplant recipients; 23 episodes of RSV | 42±13 | Inhaled ribavirin 2 g every 8 hours ×15 doses; IVIG 0.5 g/kg once; methylprednisolone 500 mg IV daily ×3 days; palivizumab IV 15 mg/kg once | No progression from URTI to LRTI occurred; no treatment episodes required mechanical ventilation |
| Tsitsikas et al. (7) | Retrospective review | Adult HSCT patients; 8 episodes of RSV | 42±12 | Inhaled ribavirin 2 g every 8 hours ×5 days; palivizumab 15 mg/kg IV | 30-day mortality: 25% (2/8); 75% of patients were RSV PCR negative after completion of treatment |
| McCoy et al. (8) | Retrospective review | Adults with hematologic malignancies and/or HSCT; 26 episodes of RSV | 51.5±11 | Inhaled ribavirin 6 g every 24 hours ×3 days; palivizumab 15 mg/kg IV once; 13 patients received ribavirin only; 13 patients received ribavirin and palivizumab | 30-day mortality: 0%; 90-day mortality: 7.7% (2/26), both patients had negative RSV antigen tests prior to their deaths |
| Grodin et al. (9) | Case report | Heart transplant recipient with URTI and LRTI | 70 | Inhaled ribavirin 2 g every 8 hours ×5 days; IVIG 0.4 mg/kg once; palivizumab 7.5 mg/kg; methylprednisolone 40 mg IV daily ×3 days | RSV antigen negative after 5 days of treatment and able to wean off bilevel positive airway pressure ventilation; 3-month follow-up CT revealed resolution of infiltrates along with improvement of pulmonary function tests |
| de Fontbrune et al. (18) | Retrospective review | Patients with allogenic HSCT; 40 episodes of RSV, 19 of which received palivizumab | 16 (IQR, 3–65) | Palivizumab 12 mg/kg IV for 1–3 injections per month vs. treatment without palivizumab | No difference in progression from URTI to LRTI (56% progression in both groups); no difference in 1-year overall survival between groups (P=0.71) |
Age is expressed as a single number for case reports, and for retrospective reviews it is expressed as mean ± standard deviation or median with interquartile range. HSCT, hematopoietic stem cell transplant; IV, intravenous; RSV, respiratory syncytial virus; IVIG, intravenous immunoglobulin; URTI, upper respiratory tract infection; LRTI, lower respiratory tract infection; PCR, polymerase chain reaction; CT, computed tomography; IQR, interquartile range.
Our case notably differs from previous literature of the use of palivizumab for adult immunocompromised patients in three ways. Firstly, oral ribavirin was utilized rather than inhaled ribavirin. In most cases where ribavirin was used in combination with palivizumab, the inhalation route was utilized (6-9). As previously mentioned, cost and side effects of this route can be limiting. Therefore, our case demonstrates that the combination of oral ribavirin with palivizumab is a viable option. Second, palivizumab was administered intramuscularly rather than intravenously. The intramuscular route is the approved route when used as prophylaxis in infants and thus was selected due to provider familiarity; however, IV administration has also been shown to be safe and efficacious (5-9). Finally, a dose of palivizumab 11 mg/kg was selected. This represents a lower dose than has been frequently utilized in literature (6-8). The infectious diseases, critical care, transplant, and pharmacy groups worked together to arrive at a dose which was likely to be efficacious and cost effective, particularly citing Grodin et al. in their treatment of a heart transplant patient with “half-dose” palivizumab (9). This dose was also driven by drug availability and rounding to the nearest vial size.
Conclusions
While there is insufficient evidence to recommend palivizumab in all immunocompromised patients with RSV, it may have some utility as salvage therapy in patients who are immunocompromised at baseline who fail to improve on more conventional therapy. This case adds to the growing, albeit limited, literature of the use of palivizumab in SOT patients and is the first to report on its use in liver transplant. The successful treatment of our patient with a lower dose of palivizumab joins previous case studies that have examined alternative dosing from 7.5–12 mg/kg, regimens which may confer significant cost savings as compared to the traditional 15 mg/kg (5,9,18). It will be prudent to revisit the utilization of this modified dosing regimen (both dose and route) in other SOT recipients. Finally, though the success from the numerous case reports and reviews collectively demonstrates promise in utilizing palivizumab more broadly for RSV in adult immunocompromised patients, the true gold-standard for demonstrating its utility would be with a randomized-controlled clinical trial (RCT). The collection of data presented in this report is encouraging for the development of an RCT at our institution.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-22-59/rc
Peer Review File: Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-22-59/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-22-59/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Keck M, Mindru C, Kalil AC, et al. Respiratory syncytial virus lower respiratory tract infection in a pediatric liver transplant recipient treated with oral ribavirin. Pediatr Transplant 2012;16:E348-51. [Crossref] [PubMed]
- Colosia AD, Yang J, Hillson E, et al. The epidemiology of medically attended respiratory syncytial virus in older adults in the United States: A systematic review. PLoS One 2017;12:e0182321. [Crossref] [PubMed]
- Collins PL, Fearns R, Graham BS. Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. Curr Top Microbiol Immunol 2013;372:3-38. [Crossref] [PubMed]
- Kodama F, Nace DA, Jump RLP. Respiratory Syncytial Virus and Other Noninfluenza Respiratory Viruses in Older Adults. Infect Dis Clin North Am 2017;31:767-90. [Crossref] [PubMed]
- Banna GL, Aversa SM, Cattelan AM, et al. Respiratory syncytial virus-related pneumonia after stem cell transplantation successfully treated with palivizumab and steroid therapy. Scand J Infect Dis 2004;36:155-7. [Crossref] [PubMed]
- Liu V, Dhillon GS, Weill D. A multi-drug regimen for respiratory syncytial virus and parainfluenza virus infections in adult lung and heart-lung transplant recipients. Transpl Infect Dis 2010;12:38-44. [Crossref] [PubMed]
- Tsitsikas DA, Oakervee H, Cavenagh JD, et al. Treatment of respiratory syncytial virus infection in haemopoietic stem cell transplant recipients with aerosolized ribavirin and the humanized monoclonal antibody palivizumab: a single centre experience. Br J Haematol 2009;146:574-6. [Crossref] [PubMed]
- McCoy D, Wong E, Kuyumjian AG, et al. Treatment of respiratory syncytial virus infection in adult patients with hematologic malignancies based on an institution-specific guideline. Transpl Infect Dis 2011;13:117-21. [Crossref] [PubMed]
- Grodin JL, Wu KS, Kitchell EE, et al. Respiratory syncytial virus pneumonia treated with lower-dose palivizumab in a heart transplant recipient. Case Rep Cardiol 2012;2012:723407. [Crossref] [PubMed]
- Palivizumab [package insert]. Gaithersburg, MD: MedImmune, LLC., 2014.
- BioFire® filmarray® respiratory 2.1 panel. BioFire Diagnostics (2022, March 2). Available online: https://www.biofiredx.com/products/the-filmarray-panels/filmarrayrp/
- Manuel O, Estabrook MAmerican Society of Transplantation Infectious Diseases Community of Practice. RNA respiratory viral infections in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019;33:e13511. [Crossref] [PubMed]
- Avery L, Hoffmann C, Whalen KM. The Use of Aerosolized Ribavirin in Respiratory Syncytial Virus Lower Respiratory Tract Infections in Adult Immunocompromised Patients: A Systematic Review. Hosp Pharm 2020;55:224-35. [Crossref] [PubMed]
- Permpalung N, Thaniyavarn T, Saullo JL, et al. Oral and Inhaled Ribavirin Treatment for Respiratory Syncytial Virus Infection in Lung Transplant Recipients. Transplantation 2020;104:1280-6. [Crossref] [PubMed]
- Foolad F, Aitken SL, Shigle TL, et al. Oral Versus Aerosolized Ribavirin for the Treatment of Respiratory Syncytial Virus Infections in Hematopoietic Cell Transplant Recipients. Clin Infect Dis 2019;68:1641-9. [Crossref] [PubMed]
- Trang TP, Whalen M, Hilts-Horeczko A, et al. Comparative effectiveness of aerosolized versus oral ribavirin for the treatment of respiratory syncytial virus infections: A single-center retrospective cohort study and review of the literature. Transpl Infect Dis 2018;20:e12844. [Crossref] [PubMed]
- Martín-Cerezuela M, Cuéllar-Monreal MJ, Monte-Boquet E, Solé-Jover A, Poveda-Andrés JL. Oral Ribavirin for Treatment of Respiratory Syncytial Virus in Lung Transplantation Recipients. Transplant Proc 2021;53:2702-5. [Crossref] [PubMed]
- de Fontbrune FS, Robin M, Porcher R, et al. Palivizumab treatment of respiratory syncytial virus infection after allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2007;45:1019-24. [Crossref] [PubMed]
- Palivizumab. Lexi-Drugs. Hudson, OH: Lexicomp, 2022. Available online: http://online.lexi.com/. Updated April 19, 2022. Accessed April 21, 2022.
- Mac S, Sumner A, Duchesne-Belanger S, et al. Cost-effectiveness of Palivizumab for Respiratory Syncytial Virus: A Systematic Review. Pediatrics 2019;143:e20184064. [Crossref] [PubMed]
Cite this article as: Labay CE, Harris JE, Saille JC, Lin J. Treatment of respiratory syncytial virus with palivizumab in an adult liver transplant recipient: a case report. J Emerg Crit Care Med 2023;7:1.


