
A severe adverse event after COVID-19 vaccination: a case report of toxic epidermal necrolysis syndrome
Highlight box
Key findings
• We reported a case of Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) in a woman 5 days after the fourth dose of the coronavirus disease 2019 (COVID-19) Pfizer Comirnaty vaccine. The patient started with non-specific symptoms and quickly developed painful skin lesions.
• To date, only 12 cases of COVID-19 vaccine-induced SJS/TEN have been reported in the literature, all with a good prognosis.
• The patient was carefully examined and point-of-care ultrasound of the soft tissues showed thickened and hyperechoic skin without subcutaneous involvement, a peculiar finding that further supports the diagnosis of SJS/TEN.
What is known and what is new?
• SJS/TEN are rare, fatal diseases of the mucous membranes caused by activation of the immune system, often as a side effect of drugs and their metabolites.
• The treatment of this disease is multidisciplinary and is based on both wound treatment and medical management in specialised departments.
• Vaccine-induced SJS/TEN is rarely reported and is not routinely categorised as a vaccine risk.
What is the implication, and what should change now?
• SJS/TEN may be associated with the COVID-19 vaccine, at least in a subset of unpredictably susceptible patients.
• COVID-19 vaccination far outweighs the risk associated with COVID-19 infection and remains the best option to combat this disease.
Introduction
Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) are rare, fatal diseases of the mucous membranes that result from activation of the immune system, often as a side effect of drugs and their metabolites (1). SJS is a less severe disease and occurs three times more frequently than TEN. The worldwide incidence is estimated to be 0.4–1.9 cases/million/year, while in Europe it is about 2–3 cases/million/year (2), with a decreasing trend over years (3). Despite females appears to be more prone to SJS/TEN, incidence does not differ in females vs. males when age is considered (4). Age, chronic kidney disease, presence of co-existing pneumonia, sepsis, and malignancies (especially of haematological origin), and human immunodeficiency virus (HIV) infection are risk factors for SJS/TEN development (3,4).
The prognosis of SJS/TEN depends largely on discontinuation of the aetiological agent, supportive treatments, and appropriate wound care (4). Nevertheless, the mortality rate is higher in TEN than SJS (50% vs. 10%) and could be influenced by age (above 70 years) and various factors such as recent radiotherapy, concomitant malignancies, systemic lupus erythematosus and renal insufficiency (5).
Medications are the most common aetiology for SJS/TEN with a possible onset within 8 weeks (typical exposure duration of 4 days to 4 weeks). More than 200 medications have been identified as triggers of SJS/TEN, including non-steroidal anti-inflammatory drugs (NSAIDs), allopurinol, anti-epileptic drugs (e.g., lamotrigine, phenytoin, carbamazepine), sulphonamides and nevirapine. Other antibiotics (e.g., cotrimoxazole, doxycycline, ciprofloxacin, amoxicillin), antineoplastic agents (e.g., vemurafenib, ipilimumab, pembrolizumab, nivoliumab, thalidomide, tamoxifen), and other drugs (e.g., pantoprazole, glucocorticoids, and terbinafine) may be potentially associated with SJS/TEN (5). However, up to 25–33% of cases cannot be attributed to drugs (6). A second most common cause of SJS/TEN is represented by infectious agents (e.g., Mycoplasma pneumoniae), especially in children (7,8). Other aetiological factors include herbal preparations, vaccines, systemic diseases, and iodine-containing contrast media; however, in more than one-third of cases, the aetiology remains unknown (4).
Genome-wide studies have identified populations at increased risk for SJS/TEN (9). The human leukocyte antigen B (HLA-B) 15:02 allele is correlated with a higher incidence of SJS/TEN in the Han Chinese population treated with carbamazepine. Similarly, the HLA-B 58:01 allele has been associated with allopurinol-induced SJS/TEN in European and Asian patients (9).
Vaccine-induced SJS/TEN is rarely reported and not routinely included among vaccine risks (8).
Here we describe the case of a 56-year-old woman who was admitted to the Emergency Department (ED) of St. Anna Hospital in Ferrara (Italy) for SJS/TEN secondary to coronavirus disease 2019 (COVID-19) Pfizer-Comirnaty vaccine. In addition, using the keywords “Stevens-Johnson syndrome”, “SJS”, “SJS/ TEN”, “Lyell syndrome”, “TEN”, and “vaccine-induced” a literature search disclosed twelve cases of SJS/TEN related to the vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV2). We present this case in accordance with the CARE reporting checklist (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-23-108/rc).
Case presentation
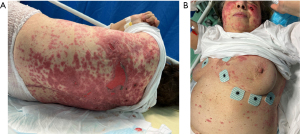
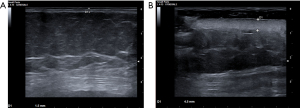
A 56-year-old woman presented at ED with fever, fatigue and rapidly developing skin lesions that occurred within 5 days after the fourth dose of COVID-19 BNT162b2 vaccine and 4 days after ribociclib. No adverse effects were reported after the previous vaccination/ribociclib administration. Her medical history revealed a metastatic ductal breast carcinoma complicated by pulmonary embolism, arterial hypertension, hypercholesterolaemia and diverticulosis. She was treated with ribociclib-letrozole, ramipril, nebivolol, and low molecular weight heparin (LMWH). Vital signs were within normal range at presentation except for body temperature (39.3 °C). The electrocardiogram (ECG) showed sinus rhythm at 100 bpm. Cardiorespiratory, abdominal, and neurological examinations were unremarkable. Skin examination showed multiple erythematous bullous lesions along with some erosions on the thorax, abdomen, face, and extremities, covering 25% of the total body surface area (BSA), with a positive Nikolsky sign (Figure 1A,1B), without mucus membrane involvement. Complete blood count, liver and kidney function tests were within normal range. C-reactive protein and procalcitonin levels were normal. Point-of-care ultrasound (PoCUS) showed a significant involvement of the skin, which appeared significantly thickened and hyperechoic in the altered compared to unaltered areas. The subcutaneous tissue, consisting of anechoic fat and discrete hyperechoic connective tissue, appeared with normal eco-structure without cobblestones. There were no areas of hypervascularisation or hypo-/anechogenicity (Figure 2A,2B).
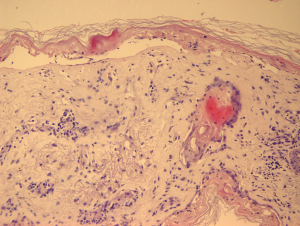
After the patient was admitted to the Department of Internal Medicine, a biopsy of the skin lesion showed widespread necrotic keratinocytes with confluent trans-epidermal necrosis and a moderate inflammatory infiltrate of the skin (Figure 3). The direct immunofluorescence test was negative, proving that the disease was not due to deposition of antibodies. Therefore, after histological exclusion of other bullous diseases, the diagnosis SJS/TEN was confirmed and the patient was referred to the regional dermatology unit specialised in severe burns. According to the dermatologist’s consult, the treatment consisted of fluids, steroids (methylprednisolone 1 to 1.5 mg/kg/day), intravenous immunoglobulin (IVIG; 500 mg/kg once daily for 4 days), tumour necrosis factor-alpha (TNF-α) inhibitors (i.e., etanercept 50 mg twice weekly) and topical skin care (with boric acid 3%, fusidic acid and betamethasone). After a period of 2 weeks, the lesions started to improve and the patient was discharged after a further week of treatment with topical bandage and moisturising gel for the skin. Treatment with ribociclib was resumed after complete resolution of the skin lesions without further symptoms.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
SJS/TEN are rare and potentially life-threatening diseases. The main pathophysiological mechanism is a delayed T-cell-mediated hypersensitivity reaction involving the skin and mucous membranes. Fas ligand (FasL) secreted by lymphocytes binds to Fas receptor expressed by keratinocytes. As a result of Fas/FasL binding granzymes and perforin are released and activate CD8 T lymphocytes leading to apoptosis of epidermal cells with detachment of the dermal-epidermal junction (10-12). In vaccine-induced SJS/TEN, apoptosis is thought to be the interaction between the virotopic antigen (expressed by the surface of keratinocytes) and the vaccine itself (10).
SJS/TEN is part of a spectrum of skin diseases that differ from BSA which is characterized by erosive blistering (10% for SJS; 30% for TEN; 10–30% in SJS/TEN overlap) (10,11). The onset may be non-specific with fever, conjunctival hyperaemia and stomatitis leading to impaired oral intake (1). Cutaneous manifestations usually occur after a few days and initially affect the anterior thoracic area, face, palms, and soles. As it progresses, SJS/TEN may affect the oral and gastrointestinal tract mucosa, genital and respiratory tract epithelium as well as the eye (1,2). Initially, the skin lesions appear as coalescing erythematous patches with purpuric centres causing severe pain that is out of proportion to the skin findings (4). As the disease progresses, vesicles and bullae may appear with a positive Nikolsky sign (i.e., detachment of the epidermis on gentle pressure on an apparently uninvolved area) (2). These epidermal manifestations resemble large burns (with the exception that only the epidermis is involved) and can cause significant fluid loss, electrolyte deficit and high risk of infections with sepsis and multiple organ failure (4). Wound management may include surgical or conservative approaches, with the latter including a biological dressing to prevent further skin abrasions (4). Since pharmacological treatments (e.g., corticosteroids, IVIG, cyclosporine, and anti-TNF-α agents) showed limited efficacy, the treatment of SJS/TEN is based only on supportive care (4).
In the present case, the skin reaction occurred 5 days after administration of the vaccine COVID-19 and 4 days after ribociclib, thus we hypothesised that there was a possible association between SJS/TEN and one of these two agents. To date, only 12 cases of COVID-19 vaccine-induced SJS/TEN have been reported in the literature (12-23) (summarised in Table 1). The age ranged from 12 to 76 years and seven of the 12 patients were female. In addition, five patients had underlying diseases (i.e., hyperlipidaemia, hypertension, diabetes mellitus, psoriasis, breast cancer and obesity). Most cases of SJS/TEN occurred after the first (12-18) or second (19-22) vaccine dose within approximately 1–2 weeks. We identified four cases attributable to Sinopharm, three to Pfizer-BioNTech, two to Moderna, two to AstraZeneca, and one to Vaxvetria. The diagnosis was confirmed by a skin biopsy in nine patients. Treatments included anti-TNF-α agents, prednisolone, cyclosporine and IVIGs. All patients had a good prognosis with complete regression between 7 days and 8 weeks. In contrast, ribociclib is a newly approved cyclin-dependent kinase inhibitor that has been associated with three cases of SJS (24).
Table 1
| Reference | Year | Age (years) | Sex | Comorbidities | Other therapies ongoing | Vaccine received | Dose | Onset symptoms after vaccine (days) | Diagnosis | Skin biopsy | Recovery time (days) | Treatment | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bakir et al. (12) | 2021 | 49 | F | No | No | Pfizer-BioNTech | First dose | 7 | TEN | Confirmed | 22 | Etanercept | Good |
| Dash et al. (13) | 2021 | 60 | M | Diabetes, hypertension | Teneligliptin, metformin, amlodipine | AstraZeneca | First dose | 3 | SJS | Confirmed | 7 | Cyclosporine | Good |
| Mardani et al. (14) | 2022 | 76 | M | Hyperlipidaemia | Atorvastatin | Sinopharm | First dose | 1 | TEN | Confirmed | 14 | Oral prednisolone | Good |
| Kherlopian et al. (15) | 2022 | 48 | F | No | No | AstraZeneca | First dose | 14 | TEN | Confirmed | 35 | Etanercept | Good |
| Mansouri et al. (16) | 2022 | 63 | F | Psoriasis, diabetes | Sitagliptin, metformin | Sinopharm | First dose | 1 | SJS | Confirmed | 21 | Oral prednisolone | Good |
| Padniewski et al. (17) | 2022 | 46 | F | Hyperlipidaemia, obesity | Metformin, atorvastatin | Moderna | First dose | 3 | SJS | Confirmed | 6 | Oral prednisolone | Good |
| Siripipattanamongkol et al. (18) | 2022 | 12 | F | No | No | Pfizer-BioNTech | First dose | 6 | TEN | Confirmed | 12 | IVIG | Good |
| Elboraey et al. (19) | 2021 | 50 | F | No | No | Pfizer-BioNTech | Second dose | 5 | SJS | Not performed | N/A | Oral prednisolone | Good |
| Aimo et al. (20) | 2022 | 65 | M | No | No | Vaxvetria | Second dose | 10 | SJS | Confirmed | Within 8 weeks |
Oral prednisolone | Good |
| Boualila et al. (21) | 2022 | 32 | M | No | No | Sinopharm | Second dose | 6 hours | SJS | Not performed | N/A | Topical corticosteroid/tobramycin, vitamins A and C, doxycycline | Good |
| Mansouri et al. (22) | 2021 | 49 | F | Breast cancer | Tamoxifen, sodium valproate, alprazolam | Sinopharm | Second dose | 3 | SJS | Confirmed | 14 | Oral prednisolone | Good |
| Battaglini et al. (23) | 2022 | 67 | M | N/A | N/A | Moderna | N/A | N/A | SJS | Not performed | N/A | N/A | N/A |
| Present case | 2023 | 56 | F | Breast cancer; PE, complicated diverticulitis, hypertension, hyperlipidaemia | Ribociclib-lerozole, ramipril, nebivolol, LMWH, allergy to Ibuprofen | Pfizer-BioNTech | Fourth dose | 5 | TEN | Confirmed | 25 | Etanercept, IVIG | Good |
TEN, toxic epidermal necrolysis; F, female; M, male; SJS, Stevens-Johnson syndrome; IVIG, intravenous immunoglobulin; N/A, not available; PE, pulmonary embolism; LMWH, low molecular weight heparin.
The correlation between these two drugs (i.e., COVID-19 vaccine BNT162b2 and ribociclib) and SJS/TEN was analysed using the Naranjo score for the probability of an adverse drug reaction, with a higher score for the vaccine than for ribociclib. Furthermore, the patient resumed the antitumour drug without any further adverse events. Therefore, it is plausible that the cause for the SJS/TEN in this case is COVID-19 vaccine although ribociclib can be definitively ruled out.
Our case presented with non-specific initial symptoms (i.e., high fever and fatigue) followed by rapidly evolving skin lesions with multiple erythematous bullae on the thorax, face and extremities affecting about 25% of the skin. Clinically relevant findings were obtained by using soft tissue ultrasound (ST-US), performed with a linear high-frequency transducer. This ultrasonographic approach identified three continuous hyperechoic structures (from top to bottom): skin, fascia and bony cortex. The skin and fascia separate the subcutaneous tissue, which consists of anechoic fat and discrete hyperechoic connective tissue. The fascia covers the muscles, which have echogenic connective tissue around hypoechoic muscle elements that look like speckled muscles on transverse scans and spindles on longitudinal scans. Tendons, ligaments and nerves show echogenic and fibrillar patterns on long-axis scans and echogenic and speckled patterns on transverse-axis scans. ST-US is a special application of POCUS that has been useful in detecting cellulitis, necrotising fasciitis, abscesses and foreign bodies (25,26). Typically, tissue infection manifests as thickening of the subcutaneous tissue, which loses its fibrillar structure and turns into a typical cobblestone appearance. In necrotising fasciitis, fluid collections under the fascia and the appearance of hyperechoic spots (free air) correlate with the diagnosis of this inflammatory condition. To our knowledge, this is the first described case of SJS/TNA by ST-US. As detectable in Figure 2, ST-US showed the exclusive involvement of the skin in the areas affected by the disease, which appeared severely thickened and hyperechoic, while the subcutaneous tissue revealed a regular structure without fluid accumulation in both the affected and the unaffected skin areas.
Conclusions
In this manuscript, we reported a case of SJS/TEN in a woman who developed fever and rapidly evolving skin lesions 5 days after receiving the fourth dose of the COVID-19 Pfizer-Comirnaty vaccine. The herein-described case showed common features with those that have been previously reported and peculiar ultrasonographic findings which corroborate that SJS/TEN may be related to COVID-19 vaccine at least in a subset of unpredictably susceptible patients. Nonetheless, the use of COVID-19 vaccination far outweighs the risk linked to COVID-19 infection and remains the best option to overcome this disease.
Acknowledgments
Funding: This study was supported by the “Fondi Ateneo per la Ricerca” (FAR) Research Fund from the University of Ferrara, Ferrara, Italy (No. 2022-FAR.L-DR_018; to R.D.G.).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-23-108/rc
Peer Review File: Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-23-108/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-23-108/coif). R.D.G. reports “Fondi Ateneo per la Ricerca” (FAR) Research Fund from the University of Ferrara, Ferrara, Italy (No. 2022-FAR.L-DR_018). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Creamer D, Walsh SA, Dziewulski P, et al. UK guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. J Plast Reconstr Aesthet Surg 2016;69:e119-53. [Crossref] [PubMed]
- Grazina I, Mannocci A, Meggiolaro A, et al. Is there an association between Stevens-Johnson Syndrome and vaccination? A systematic review. Ann Ig 2020;32:81-96. [PubMed]
- Wasuwanich P, So JM, Chakrala TS, et al. Epidemiology of Stevens-Johnson syndrome and toxic epidermal necrolysis in the United States and factors predictive of outcome. JAAD Int 2023;13:17-25. [Crossref] [PubMed]
- Charlton OA, Harris V, Phan K, et al. Toxic Epidermal Necrolysis and Steven-Johnson Syndrome: A Comprehensive Review. Adv Wound Care (New Rochelle) 2020;9:426-39. [Crossref] [PubMed]
- Knowles S, Shear NH. Clinical risk management of Stevens-Johnson syndrome/toxic epidermal necrolysis spectrum. Dermatol Ther 2009;22:441-51. [Crossref] [PubMed]
- Sassolas B, Haddad C, Mockenhaupt M, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther 2010;88:60-8. [Crossref] [PubMed]
- Meyer Sauteur PM, Goetschel P, Lautenschlager S. Mycoplasma pneumoniae and mucositis--part of the Stevens-Johnson syndrome spectrum. J Dtsch Dermatol Ges 2012;10:740-6. [Crossref] [PubMed]
- Vujic I, Shroff A, Grzelka M, et al. Mycoplasma pneumoniae-associated mucositis--case report and systematic review of literature. J Eur Acad Dermatol Venereol 2015;29:595-8. [Crossref] [PubMed]
- Dodiuk-Gad RP, Chung WH, Valeyrie-Allanore L, et al. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: An Update. Am J Clin Dermatol 2015;16:475-93. [Crossref] [PubMed]
- Ball R, Ball LK, Wise RP, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis after vaccination: reports to the vaccine adverse event reporting system. Pediatr Infect Dis J 2001;20:219-23. [Crossref] [PubMed]
- Grünwald P, Mockenhaupt M, Panzer R, et al. Erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis - diagnosis and treatment. J Dtsch Dermatol Ges 2020;18:547-53. [Crossref]
- Bakir M, Almeshal H, Alturki R, et al. Toxic Epidermal Necrolysis Post COVID-19 Vaccination - First Reported Case. Cureus 2021;13:e17215. [Crossref] [PubMed]
- Dash S, Sirka CS, Mishra S, et al. COVID-19 vaccine-induced Stevens-Johnson syndrome. Clin Exp Dermatol 2021;46:1615-7. [Crossref] [PubMed]
- Mardani M, Mardani S, Asadi Kani Z, et al. An extremely rare mucocutaneous adverse reaction following COVID-19 vaccination: Toxic epidermal necrolysis. Dermatol Ther 2022;35:e15416. [PubMed]
- Kherlopian A, Zhao C, Ge L, et al. A case of toxic epidermal necrolysis after ChAdOx1 nCov-19 (AZD1222) vaccination. Australas J Dermatol 2022;63:e93-5. [Crossref] [PubMed]
- Mansouri P, Farshi S. A case of Steven-Johnson syndrome after COVID-19 vaccination. J Cosmet Dermatol 2022;21:1358-60. [Crossref] [PubMed]
- Padniewski JJ, Jacobson-Dunlop E, Albadri S, et al. Stevens-Johnson syndrome precipitated by Moderna Inc. COVID-19 vaccine: a case-based review of literature comparing vaccine and drug-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. Int J Dermatol 2022;61:923-9. [Crossref] [PubMed]
- Siripipattanamongkol N, Rattanasak S, Taiyaitieng C, et al. Toxic epidermal necrolysis after first dose of Pfizer-BioNTech (BNT162b2) vaccination with pharmacogenomic testing. Pediatr Dermatol 2022;39:601-5. [Crossref] [PubMed]
- Elboraey MO, Essa EESF. Stevens-Johnson syndrome post second dose of Pfizer COVID-19 vaccine: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol 2021;132:e139-42. [Crossref] [PubMed]
- Aimo C, Mariotti EB, Corrà A, et al. Stevens-Johnson syndrome induced by Vaxvetria (AZD1222) COVID-19 vaccine. J Eur Acad Dermatol Venereol 2022;36:e417-9. [Crossref] [PubMed]
- Boualila L, Mrini B, Tagmouti A, et al. Sinopharm COVID-19 vaccine-induced Stevens-Johnson syndrome. J Fr Ophtalmol 2022;45:e179-82. [Crossref] [PubMed]
- Mansouri P, Chalangari R, Martits-Chalangari K, et al. Stevens-Johnson Syndrome due to COVID-19 vaccination. Clin Case Rep 2021;9:e05099. [Crossref] [PubMed]
- Battaglini D, Ball L, Robba C, et al. Patients With Suspected Severe Adverse Reactions to COVID-19 Vaccination Admitted to Intensive Care Unit: A Case Report. Front Med (Lausanne) 2022;9:823837. [Crossref] [PubMed]
- Silvestri M, Cristaudo A, Morrone A, et al. Emerging Skin Toxicities in Patients with Breast Cancer Treated with New Cyclin-Dependent Kinase 4/6 Inhibitors: A Systematic Review. Drug Saf 2021;44:725-32. [Crossref] [PubMed]
- Gottlieb M, Avila J, Chottiner M, et al. Point-of-Care Ultrasonography for the Diagnosis of Skin and Soft Tissue Abscesses: A Systematic Review and Meta-analysis. Ann Emerg Med 2020;76:67-77. Erratum in: Ann Emerg Med 2022;79:90. [Crossref] [PubMed]
- Chen KC, Lin AC, Chong CF, et al. An overview of point-of-care ultrasound for soft tissue and musculoskeletal applications in the emergency department. J Intensive Care 2016;4:55. [Crossref] [PubMed]
Cite this article as: Guarino M, Benedetto M, Blanaru OT, Perna B, Costanzini A, Previati R, Spampinato MD, De Giorgio R. A severe adverse event after COVID-19 vaccination: a case report of toxic epidermal necrolysis syndrome. J Emerg Crit Care Med 2024;8:4.


