
Biomarkers for unfavourable outcomes prediction in COVID-19 patients: a narrative review
Introduction
Since SARS-CoV-2 was first identified, it has rapidly spread around the world, causing a pandemic (1). As of 22 November 2023, WHO reported over 772 million confirmed cases of coronavirus disease 2019 (COVID-19) and almost 7 million deaths worldwide (2). Also, COVID-19 significantly impacts the social, economic, and psychological spheres of human life (3). COVID-19 may be asymptomatic or variable symptomatic from mild symptoms such as cough, sore throat, high temperature, diarrhoea, headache, muscle or joint pain, fatigue, and loss of sense of smell and taste (4) to severe acute respiratory syndrome and multiorgan failure (5,6).
Multiple biomarkers for the prediction of severe COVID-19 and mortality have been studied. Biomarkers are important for early suspicion of unfavourable outcomes, rationalization of patient management, and assessment of response to treatment. Knowledge about biomarkers of unfavourable outcome for COVID-19 is very important for any doctor treating patients with COVID-19. We present this article in accordance with the Narrative Review reporting checklist (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-58/rc).
Methods
This review is aimed to summarize available data about biomarkers of unfavourable outcomes in COVID-19 patients and classify them. Literature search was performed through the scientific database PubMed and assessed literature from 2019 to March 2024. The most relevant studies and meta-analyses about biomarkers predicting COVID-19 severity and mortality were reviewed. Also, these biomarkers were classified. For further details about search method see Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | December 2023 to March 2024 |
| Databases and other sources searched | PubMed |
| Search terms used | COVID-19 OR coronavirus disease* |
| * AND biomarkers OR biomarker | |
| * AND prediction OR predict | |
| * AND severity OR mortality OR unfavorable outcome OR death | |
| Timeframe | 2019 to 2024 |
| Inclusion and exclusion criteria | Inclusion criteria: observational studies, narrative reviews, systematic reviews and meta-analyses |
| Exclusion criteria: (I) case reports and case series; (II) non-English language | |
| Selection process | At first, each author independently reviewed the literature and wrote a specific section(s) of the paper. Then, all authors have read the article and suggest some changes. Finally, when consensus among all authors was obtained the article was submitted |
COVID-19, coronavirus disease 2019.
Laboratory parameters predicting unfavourable outcome in COVID-19
Hematological parameters
Haemoglobin level tends to be lower in patients with severe disease (7,8). Anemia is associated with about 70% higher risk of short-term mortality in COVID-19 patients (9). Higher white blood cell count at hospital admission is associated with higher risk of death in COVID-19 (10). The immunological phenotype of severe COVID-19 is characterized by elevated neutrophil count and reduced lymphocyte count (11). Lymphopenia is associated with the disease severity and mortality (12,13). As there is reduced lymphocyte count and elevated neutrophil count in COVID-19 patients, the neutrophil-lymphocyte ratio (NLR) increases with the severity of the disease. It was found that the NLR of 9.47 is the optimal cut-off value for predicting mortality and the NLR of 5.86 is the optimal cut-off value for predicting severity (14). Thrombocytopenia is another important marker of disease severity and mortality (15). Patients with thrombocytopenia have 7-fold higher odds of mortality from COVID-19 (16). There are following proposed mechanisms of thrombocytopenia in COVID-19 patients: (I) inhibition of platelet production in bone marrow by direct injury of hematopoietic and bone marrow stromal cells or mediated by cytokine storm; (II) platelet destruction caused by increase of autoantibodies and immune complexes; (III) decrease in circulating platelet due to platelet activation, aggregation and wrapping into microthrombus (17). Also, absolute eosinopenia is found to be a predictor of severe COVID-19 and death (18). Systemic immune-inflammation index (SII) calculated based on the complete blood parameters (neutrophils × platelets/lymphocytes) is an important proinflammatory marker of systemic inflammation that can be effectively used as an independent prediction of mortality in COVID-19 patients with an optimal cut-off value of 618.8 (19). Platelet-lymphocyte ratio (PLR) is another reliable marker of severity and mortality among patients with COVID-19 (20). Mean platelet volume (MPV) and platelet distribution width (PDW) are elevated in COVID-19 patients compared to non-COVID individuals (21) and may be used as predictors of mortality (22,23). High platelet-large cell ratio is associated with mortality (24).
Inflammatory markers
COVID-19 may lead to hyperinflammatory response and ‘cytokine storm’ and tissue damage via apoptosis and pyroptosis (25). So, inflammatory markers may reflect the severity of the disease. It’s known that IL-6 is elevated in COVID-19 patients (26) and may be used as a predictor of severe COVID-19 (27) and in-hospital mortality (28). Higher C-reactive protein (CRP) levels are associated with disease progression and mortality (29). The optimal cut-off value of CRP for prediction of severe complications is 64.75 mg/dL (30). Patients with high tumour necrosis factor (TNF) and IL-23 on admission are more likely to experience a severe form of COVID-19 (31). Serum amyloid A levels are positively associated with the severe disease and mortality (32). Procalcitonin is another marker reflecting an inflammatory state that has good discriminative power for predicting mortality and disease severity in COVID-19 patients (33). Elevated procalcitonin may reflect hyperinflammatory condition (34). Erythrocyte sedimentation rate is found to be higher among those with pneumonia, requiring oxygen, and non-survivors, but its prognostic value is poor (35). Also, elevated lactate dehydrogenase is associated with a poor outcome in COVID-19 (36,37). Granulocyte-macrophage colony-stimulating factor, C-X-C motif chemokine ligand 10 (CXCL10), IL-1β, IL-8, IL-15, resistin, macrophage inflammatory protein-1α (MIP-1α) and intracellular adhesion molecule 1 (ICAM-1), monocyte chemoattractant protein 1 (MCP-1) were associated with adverse outcomes (38-43). IL-10 is an anti-inflammatory cytokine that is a reliable predictor of severity and mortality in COVID-19 patients (44). Also, a low level of amino-terminal propeptide of C-type natriuretic peptide (NT-proCNP) on hospital admission is associated with severe COVID-19 (45) and predicts the severe disease (46).
Elevated soluble IL-2 receptor (sIL-2R) level suggests a hyper-inflammatory state reflecting the severity of COVID-19 and may be used as a predictor of severity and mortality (47-49). sIL-2R are secreted by activated T-cells and their elevated levels are a marker for T-cell activity (50,51).
Transforming growth factor-β1, an important immunomodulatory and pro-fibrotic cytokine is found to be an efficient biomarker for predicting COVID-19 severity and adverse outcomes in patients with non-alcoholic fatty liver disease (52). Patients who died of severe COVID-19 have significantly higher levels of transforming growth factor-β at the disease onset and this biomarker may be used as a predictor of fatal outcomes [area under the curve (AUC), 0.75] (53).
Ferritin is one of the most important biomarkers used as a predictor of unfavourable outcomes in COVID-19 patients. According to the FerVid study, a ferritin level of >500 ng/mL is found in more than 50% of COVID-19 patients (54). Ferritin values >3,000 ng/mL are referred to hyperferritinemic syndrome and have high specificity (96%) for unfavourable outcomes (54). Ferritin levels at admission may serve as a predictor of ICU admission, the need for mechanical ventilation and in-hospital mortality (55,56). However, as ferritin level is lower in the female than in male patients with COVID-19, the optimal cut-off values are different too (57). The optimal ferritin cut-off values for mortality were 433 ng/mL in women and 740 ng/mL in men (58). To improve predictive abilities of ferritin, ferritin-hemoglobin ratio (59), ferritin-albumin ratio (60), and ferritin-lymphocyte ratio (61) were studied.
Iron metabolism
Hepcidin is an important iron-regulating protein (62) that was found to be a good biomarker in predicting the severity and mortality of COVID-19 in hospitalized (63) and ICU patients (64). Hepcidin blocks iron export from cells through ferroportin, a transmembrane protein that exports iron from duodenal enterocytes absorbing dietary iron, from iron-recycling macrophages in the spleen and the liver, and from iron-storing hepatocytes (65). Patients with COVID-19 present with lower serum iron levels that may be explained by increased iron internalization due to reduced iron export from cells via ferroportin (66). Serum iron level is an accurate predictor of hospitalization (optimal cut-off value of 6.0 µmol/L with a sensitivity of 94.7% and a specificity of 67.9%; AUC, 0.894) (67). Also, serum iron showed good predictive abilities for COVID-19 severity (optimal cut-off value, 5.26 µmol/L; sensitivity, 58.1%; specificity, 89.5%; AUC, 0.696) and mortality (optimal cut-off value, 12.54 µmol/L; sensitivity, 100.0%; specificity, 77.8%; AUC, 0.929) (68). Reduced serum transferrin levels reflect heightened inflammation in COVID-19 patients and are an important predictor of the severity and progression of the disease (69). So, COVID-19 patients present with low levels of transferrin, iron, and haemoglobin, as well as high ferritin and hepcidin levels.
Lipid and glucose profile
Low total cholesterol, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein cholesterol levels and high triglycerides are associated with severe COVID-19 patients and mortality (70-72). To improve the predictive abilities of the lipid spectrum, triglyceride-HDL ratio (73), and monocyte-HDL ratio (74) were studied and had predictive abilities.
It’s known that both diabetes mellitus, hyperglycaemia, and elevated haemoglobin A1c levels are independently associated with adverse prognosis of COVID-19 (75-77).
Coagulation status
Often coagulation abnormalities are seen in COVID-19 patients. Causal factors of COVID-19-associated coagulopathy include inflammation, endothelial dysfunction, platelet and complement activation, renin-angiotensin-aldosterone system derangement, and hypoxemia (78). Patients with COVID-19 have higher values of thrombin-antithrombin complex, α2-plasmin inhibitor-plasmin complex, thrombomodulin, t-PA/PAI-1 complex, prothrombin time, international normalized ratio, fibrinogen, thrombin time, and D-dimer (79). Prothrombin time is found to be a predictor of the disease severity and mortality, an increase in prothrombin time is associated with worsened prognosis (80). An optimal cut-off value of prothrombin time was 16.25 seconds for severe COVID-19 (80). Also, γ′ fibrinogen is a useful inflammatory marker of COVID-19 respiratory disease severity (81). Activated partial thromboplastin time >27.1 seconds may be used as a predictor of mortality in COVID-19 patients (82). Elevated levels of Willebrand factor antigen and soluble thrombomodulin representing endotheliopathy are associated with mortality (83). Patients with a D-dimer level greater than 0.5 µg/mL have 5.78 times higher odds of severe COVID-19 (84). 1.5 µg/mL is the optimal cut-off value of D-dimer for predicting mortality in COVID-19 patients (sensitivity, 70.6%; specificity, 78.4%) (5).
Markers of organ dysfunction
In addition to pulmonary involvement, COVID-19 may impair cardiac, liver and kidney function (85). N-terminal pro-brain natriuretic peptide (NT-proBNP), the most studied marker of heart failure, is found to be an independent risk factor for in-hospital death in patients with severe COVID-19; its optimal cut-off value is 88.64 pg/mL (86). High levels of mid-regional pro-atrial natriuretic peptide (MR-proANP) at admission are associated with COVID-19 severity and appeared to be an independent prognostic marker of 28-day mortality (AUC, 0.832) (87). Patients with elevated troponin I levels have 7.92 higher odds of poor outcomes compared to patients with normal troponin levels (88). Soluble suppression of tumorigenesis-2 (sST2), a biomarker of heart failure, is a useful biomarker to predict ICU admission, ventilator use, extracorporeal membrane oxygenation use, and 30-day mortality in hospitalized COVID-19 patients (89).
Elevated creatine kinase level is found to be associated with severity of disease, even when adjusting for CRP (90). Myoglobin predicts mortality in severe/critical COVID-19 patients and is found to be superior to troponin for predictive value (91).
Elevated alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and total bilirubin levels, as well as decreased albumin levels, are associated with unfavourable outcomes in COVID-19 patients (92).
Elevated creatinine, blood urea nitrogen, and blood urea nitrogen/creatinine ratio are associated with COVID-19 severity and lethal outcomes (93,94). Severity of COVID-19 may be predicted by blood urea nitrogen/albumin ratio (cut-off value, 6.23 mg/g; sensitivity, 79%; specificity, 54%, AUC, 0.695) (95). Serum cystatin C is a predictor of severe COVID-19; the cut-off value is 1.245 mg/L (sensitivity, 79.1%; specificity, 60.7%) (96).
Krebs von den Lungen-6 (KL-6) glycoprotein expressed on type II alveolar epithelium is found to be higher in patients with severe COVID-19 and may be used as a predictor of the severe disease representing lung injury (97,98).
Biomarkers of unfavourable outcomes in COVID-19 in clinical practice
Summarizing the data of these studies, published studies showed that COVID-19 may be accompanied by pulmonary, cardiovascular, hepatic, and renal involvement that is associated with unfavourable outcomes. Also, plenty of biomarkers representing inflammation and coagulation states, iron, lipid, and glucose metabolism, as well as haematological changes may be used as predictors of unfavourable outcomes in COVID-19 patients (Figure 1).
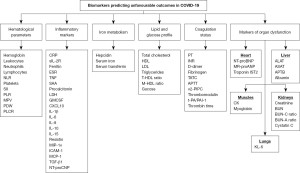
Despite the fact that multiple biomarkers of unfavourable outcomes in COVID-19 have been studied, plenty of them are expensive or unavailable in clinics. Also, predictive abilities of some biomarkers are not high enough. These factors make some laboratory parameters to be secondary for unfavourable outcomes prediction.
Considering availability, cost and predictive ability, the most relevant biomarkers in real clinical practice include CRP, ferritin, IL-6, procalcitonin, and coagulation status that should be performed in addition to routine laboratory tests. If there are symptoms, signs or any other evidence of organ dysfunction specific laboratory tests and investigations should be performed.
Conclusions
Thus, biomarkers predicting unfavourable outcomes in COVID-19 patients may be classified as haematological parameters, inflammatory markers, markers of iron metabolism, lipid and glucose profile, coagulation status, and markers of organ dysfunction. As each biomarker reflects a specific aspect of COVID-19 pathology, laboratory parameters may be used to guide the selection of an appropriate method of therapeutic intervention.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-58/rc
Peer Review File: Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-58/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-58/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed 2020;91:157-60. [PubMed]
- World Health Organization. WHO COVID-19 dashboard [Internet]. Available online: https://covid19.who.int/ (2023, accessed 01.12.2023).
- Mulugeta T, Tadesse E, Shegute T, et al. COVID-19: socio-economic impacts and challenges in the working group. Heliyon 2021;7:e07307. [Crossref] [PubMed]
- Struyf T, Deeks JJ, Dinnes J, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database Syst Rev 2022;5:CD013665. [PubMed]
- Zhao W, Li H, Li J, et al. The mechanism of multiple organ dysfunction syndrome in patients with COVID-19. J Med Virol 2022;94:1886-92. [Crossref] [PubMed]
- Hussain M, Khurram Syed S, Fatima M, et al. Acute Respiratory Distress Syndrome and COVID-19: A Literature Review. J Inflamm Res 2021;14:7225-42. [Crossref] [PubMed]
- Lippi G, Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther 2020;42:116-7. [Crossref] [PubMed]
- Chaudhry ZR, Rasheed S, Shakir S, et al. Corona virus lowers hemoglobin more in severe infection than mild COVID-19 infection. The Professional Medical Journal 2021;28:1211-4. [Crossref]
- Zuin M, Rigatelli G, Quadretti L, et al. Prognostic Role of Anemia in COVID-19 Patients: A Meta-Analysis. Infect Dis Rep 2021;13:930-7. [Crossref] [PubMed]
- Zhu B, Feng X, Jiang C, et al. Correlation between white blood cell count at admission and mortality in COVID-19 patients: a retrospective study. BMC Infect Dis 2021;21:574. [Crossref] [PubMed]
- McKenna E, Wubben R, Isaza-Correa JM, et al. Neutrophils in COVID-19: Not Innocent Bystanders. Front Immunol 2022;13:864387. [Crossref] [PubMed]
- Toori KU, Qureshi MA, Chaudhry A. Lymphopenia: A useful predictor of COVID-19 disease severity and mortality. Pak J Med Sci 2021;37:1984-8. [Crossref] [PubMed]
- Niu J, Sareli C, Mayer D, et al. Lymphopenia as a Predictor for Adverse Clinical Outcomes in Hospitalized Patients with COVID-19: A Single Center Retrospective Study of 4485 Cases. J Clin Med 2022;11:700. [Crossref] [PubMed]
- Tadesse Z, Bekele Bayissa A, Diriba T, et al. Neutrophil-to-Lymphocyte Ratio and Cut-off Values as Predictor of Severity and Mortality in COVID-19 Patients in Millennium COVID-19 Care Center, Addis Ababa, Ethiopia. Int J Gen Med 2022;15:6739-55. [Crossref] [PubMed]
- El-Khaiat MM, El-Lehlah AM, Kesheita MA, et al. Association between thrombocytopenia and the severity of Covid-19 infection among hospitalized Egyptian patients. Ann Med Surg (Lond) 2022;79:103973. [Crossref] [PubMed]
- Zong X, Gu Y, Yu H, et al. Thrombocytopenia Is Associated with COVID-19 Severity and Outcome: An Updated Meta-Analysis of 5637 Patients with Multiple Outcomes. Lab Med 2021;52:10-5. [Crossref] [PubMed]
- Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol 2020;99:1205-8. [Crossref] [PubMed]
- Gambichler T, Schuleit N, Susok L, et al. Prognostic Performance of Inflammatory Biomarkers Based on Complete Blood Counts in COVID-19 Patients. Viruses 2023;15:1920. [Crossref] [PubMed]
- Karaaslan T, Karaaslan E. Predictive Value of Systemic Immune-inflammation Index in Determining Mortality in COVID-19 Patients. J Crit Care Med (Targu Mures) 2022;8:156-64. [Crossref] [PubMed]
- Ravindra R, Ramamurthy P, Aslam S SM, et al. Platelet Indices and Platelet to Lymphocyte Ratio (PLR) as Markers for Predicting COVID-19 Infection Severity. Cureus 2022;14:e28206. [Crossref] [PubMed]
- Shankaralingappa A, Tummidi S, Arun Babu T. Diagnostic value of platelet indices in COVID 19 infection: a case-control study from a single tertiary care center. Egypt J Intern Med 2022;34:35. [Crossref] [PubMed]
- Bommenahalli Gowda S, Gosavi S, Ananda Rao A, et al. Prognosis of COVID-19: Red Cell Distribution Width, Platelet Distribution Width, and C-Reactive Protein. Cureus 2021;13:e13078. [Crossref] [PubMed]
- Beceren NG, Armağan HH, Oğuzlar FÇ, et al. Can mean platelet volume be a prognosis predictor in viral infections: An example of Covid-19. Heliyon 2023;9:e21983. [Crossref] [PubMed]
- Ergenç Z, Kaya G, Ocak OK, et al. Relationship of platelet subgroups with prognosis and mortality in patients with mild, severe and critical COVID-19. Hippocrates Med J 2023;3:76-81. [Crossref]
- Silva MJA, Ribeiro LR, Gouveia MIM, et al. Hyperinflammatory Response in COVID-19: A Systematic Review. Viruses 2023;15:553. [Crossref] [PubMed]
- Faraj SS, Jalal PJ. IL1β, IL-6, and TNF-α cytokines cooperate to modulate a complicated medical condition among COVID-19 patients: case-control study. Ann Med Surg (Lond) 2023;85:2291-7. [Crossref] [PubMed]
- Mojtabavi H, Saghazadeh A, Rezaei N. Interleukin-6 and severe COVID-19: a systematic review and meta-analysis. Eur Cytokine Netw 2020;31:44-9. [Crossref] [PubMed]
- Skakun O, Fedorov S, Seredyuk N, et al. Prognostic Value of Serum Interleukin-6 Level in Hypertensive Patients with COVID-19-Associated Pneumonia. Galician Med J 2022;29:E202242. [Crossref]
- Chi L, Wang S, Wang X, et al. Predictive value of C-reactive protein for disease severity and survival in COVID-19 patients: a systematic review and meta-analysis. Clin Exp Med 2023;23:2001-8. [Crossref] [PubMed]
- Sadeghi-Haddad-Zavareh M, Bayani M, Shokri M, et al. C-Reactive Protein as a Prognostic Indicator in COVID-19 Patients. Interdiscip Perspect Infect Dis 2021;2021:5557582. [Crossref] [PubMed]
- Smail SW, Babaei E, Amin K, et al. Serum IL-23, IL-10, and TNF-α predict in-hospital mortality in COVID-19 patients. Front Immunol 2023;14:1145840. [Crossref] [PubMed]
- Zinellu A, Paliogiannis P, Carru C, et al. Serum amyloid A concentrations, COVID-19 severity and mortality: An updated systematic review and meta-analysis. Int J Infect Dis 2021;105:668-74. [Crossref] [PubMed]
- Kumar A, Karn E, Trivedi K, et al. Procalcitonin as a predictive marker in COVID-19: A systematic review and meta-analysis. PLoS One 2022;17:e0272840. [Crossref] [PubMed]
- Tong-Minh K, van der Does Y, Engelen S, et al. High procalcitonin levels associated with increased intensive care unit admission and mortality in patients with a COVID-19 infection in the emergency department. BMC Infect Dis 2022;22:165. [Crossref] [PubMed]
- Kurt C, Altunçeki Ç, Yildirim A. Contribution of Erythrocyte Sedimentation Rate to Predict Disease Severity and Outcome in COVID-19 Patients. Can J Infect Dis Med Microbiol 2022;2022:6510952. [Crossref] [PubMed]
- Fialek B, Pruc M, Smereka J, et al. Diagnostic value of lactate dehydrogenase in COVID-19: A systematic review and meta-analysis. Cardiol J 2022;29:751-8. [Crossref] [PubMed]
- Mao M, Dian Y, Sun Y, et al. Lactate dehydrogenase predicts disease progression outcome in COVID-19 patients treated with Azvudine. Front Cell Infect Microbiol 2023;13:1237277. [Crossref] [PubMed]
- Blot M, Bour JB, Quenot JP, et al. The dysregulated innate immune response in severe COVID-19 pneumonia that could drive poorer outcome. J Transl Med 2020;18:457. [Crossref] [PubMed]
- Yudhawati R, Sakina S, Fitriah M. Interleukin-1β and Interleukin-10 Profiles and Ratio in Serum of COVID-19 Patients and Correlation with COVID-19 Severity: A Time Series Study. Int J Gen Med 2022;15:8043-54. [Crossref] [PubMed]
- Li L, Li J, Gao M, et al. Interleukin-8 as a Biomarker for Disease Prognosis of Coronavirus Disease-2019 Patients. Front Immunol 2020;11:602395. [Crossref] [PubMed]
- Perpiñan C, Bertran L, Terra X, et al. Resistin and IL-15 as Predictors of Invasive Mechanical Ventilation in COVID-19 Pneumonia Irrespective of the Presence of Obesity and Metabolic Syndrome. J Pers Med 2022;12:391. [Crossref] [PubMed]
- Hamza AM, Ali WDK, Hassanein N, et al. Relation between macrophage inflammatory protein-1 and intercellular adhesion molecule-1 and computed tomography findings in critically-ill saudi covid-19 patients. J Infect Public Health 2022;15:1497-502. [Crossref] [PubMed]
- Mulla S, Molla MMA, Ahmed SMA, et al. Association of interferon gamma inducible protein-10, monocyte chemoattractant protein-1, macrophage inflammatory protein-1 alpha, interleukin-6, and rs12252 single nucleotide polymorphism of interferon-induced transmembrane protein-3 gene with the severity of COVID-19 infection. Egypt J Intern Med 2022;34:53. [Crossref] [PubMed]
- Alshammary AF, Alsughayyir JM, Alharbi KK, et al. T-Cell Subsets and Interleukin-10 Levels Are Predictors of Severity and Mortality in COVID-19: A Systematic Review and Meta-Analysis. Front Med (Lausanne) 2022;9:852749. [Crossref] [PubMed]
- Bojti I, Przewosnik AS, Luxenburger H, et al. Decreased level of serum NT-proCNP associates with disease severity in COVID-19. Respir Res 2023;24:174. [Crossref] [PubMed]
- Karsli E, Anabarli Metin D, Canacik O, et al. Galectin-3 as a Potential Prognostic Biomarker for COVID-19 Disease: A Case-Control Study. Cureus 2022;14:e28805. [Crossref] [PubMed]
- Jang HJ, Leem AY, Chung KS, et al. Soluble IL-2R Levels Predict in-Hospital Mortality in COVID-19 Patients with Respiratory Failure. J Clin Med 2021;10:4242. [Crossref] [PubMed]
- Gatselis NK, Lygoura V, Lyberopoulou A, et al. Soluble IL-2R Levels at Baseline Predict the Development of Severe Respiratory Failure and Mortality in COVID-19 Patients. Viruses 2022;14:787. [Crossref] [PubMed]
- Kaya H, Kaji M, Usuda D. Soluble interleukin-2 receptor levels on admission associated with mortality in coronavirus disease 2019. Int J Infect Dis 2021;105:522-4. [Crossref] [PubMed]
- Karim AF, Eurelings LEM, Bansie RD, et al. Soluble Interleukin-2 Receptor: A Potential Marker for Monitoring Disease Activity in IgG4-Related Disease. Mediators Inflamm 2018;2018:6103064. [Crossref] [PubMed]
- Dik WA, Heron M. Clinical significance of soluble interleukin-2 receptor measurement in immune-mediated diseases. Neth J Med 2020;78:220-31. [PubMed]
- Susak F, Vrsaljko N, Vince A, et al. TGF Beta as a Prognostic Biomarker of COVID-19 Severity in Patients with NAFLD-A Prospective Case-Control Study. Microorganisms 2023;11:1571. [Crossref] [PubMed]
- Frischbutter S, Durek P, Witkowski M, et al. Serum TGF-β as a predictive biomarker for severe disease and fatality of COVID-19. Eur J Immunol 2023;53:e2350433. [Crossref] [PubMed]
- Para O, Caruso L, Pestelli G, et al. Ferritin as prognostic marker in COVID-19: the FerVid study. Postgrad Med 2022;134:58-63. [Crossref] [PubMed]
- Shakaroun DA, Lazar MH, Horowitz JC, et al. Serum Ferritin as a Predictor of Outcomes in Hospitalized Patients with Covid-19 Pneumonia. J Intensive Care Med 2023;38:21-6. [Crossref] [PubMed]
- Ahmed S, Ansar Ahmed Z, Siddiqui I, et al. Evaluation of serum ferritin for prediction of severity and mortality in COVID-19- A cross sectional study. Ann Med Surg (Lond) 2021;63:102163. [Crossref] [PubMed]
- Hadi JM, Mohammad HM, Ahmed AY, et al. Investigation of Serum Ferritin for the Prediction of COVID-19 Severity and Mortality: A Cross-Sectional Study. Cureus 2022;14:e31982. [Crossref] [PubMed]
- Qeadan F, Tingey B, Gu LY, et al. Prognostic Values of Serum Ferritin and D-Dimer Trajectory in Patients with COVID-19. Viruses 2021;13:419. [Crossref] [PubMed]
- Raman N, Kv P, Ashta KK, et al. Ferritin and Hemoglobin as Predictors of Fatal Outcome in COVID-19: Two Sides of the Same Coin. J Assoc Physicians India 2021;69:11-2. [PubMed]
- Taşkin Ö, Yilmaz A, Soylu VG, et al. Ferritin / albumin ratio could be a new indicator of COVID-19 disease mortality. J Infect Dev Ctries 2023;17:37-42. [Crossref] [PubMed]
- Liu A, Hammond R, Chan K, et al. Characterisation of Ferritin-Lymphocyte Ratio in COVID-19. Biomedicines 2023;11:2819. [Crossref] [PubMed]
- Hepcidin Ganz T. Rinsho Ketsueki 2016;57:1913-7. [PubMed]
- Nai A, Lorè NI, Pagani A, et al. Hepcidin levels predict Covid-19 severity and mortality in a cohort of hospitalized Italian patients. Am J Hematol 2021;96:E32-5. [Crossref] [PubMed]
- Ciotti M, Nuccetelli M, Pieri M, et al. Evaluation of Hepcidin Level in COVID-19 Patients Admitted to the Intensive Care Unit. Diagnostics (Basel) 2022;12:2665. [Crossref] [PubMed]
- Nemeth E, Ganz T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int J Mol Sci 2021;22:6493. [Crossref] [PubMed]
- Gaiatto ACM, Bibo TA, de Godoy Moreira N, et al. COVID-19 compromises iron homeostasis: Transferrin as a target of investigation. J Trace Elem Med Biol 2023;76:127109. [Crossref] [PubMed]
- Hippchen T, Altamura S, Muckenthaler MU, et al. Hypoferremia is Associated With Increased Hospitalization and Oxygen Demand in COVID-19 Patients. Hemasphere 2020;4:e492. [Crossref] [PubMed]
- Zhao K, Huang J, Dai D, et al. Serum Iron Level as a Potential Predictor of Coronavirus Disease 2019 Severity and Mortality: A Retrospective Study. Open Forum Infect Dis 2020;7:ofaa250. [Crossref] [PubMed]
- Claise C, Saleh J, Rezek M, et al. Low transferrin levels predict heightened inflammation in patients with COVID-19: New insights. Int J Infect Dis 2022;116:74-9. [Crossref] [PubMed]
- Masana L, Correig E, Ibarretxe D, et al. Low HDL and high triglycerides predict COVID-19 severity. Sci Rep 2021;11:7217. [Crossref] [PubMed]
- Mahat RK, Rathore V, Singh N, et al. Lipid profile as an indicator of COVID-19 severity: A systematic review and meta-analysis. Clin Nutr ESPEN 2021;45:91-101. [Crossref] [PubMed]
- Aydın SŞ, Aksakal E, Aydınyılmaz F, et al. Relationship Between Blood Lipid Levels and Mortality in Hospitalized COVID-19 Patients. Angiology 2022;73:724-33. [Crossref] [PubMed]
- Peng F, Lei S, Zhang Q, et al. Triglyceride/High-Density Lipoprotein Cholesterol Ratio is Associated with the Mortality of COVID-19: A Retrospective Study in China. Int J Gen Med 2022;15:985-96. [Crossref] [PubMed]
- Argun D, Argun F, Basim P, et al. Monocyte-to-High-Density Lipoprotein Cholesterol Ratio: a Candidate Parameter for a Risk Assessment Model in COVID-19. Clin Lab 2022; [Crossref] [PubMed]
- Long H, Li J, Li R, et al. Plasma glucose levels and diabetes are independent predictors for mortality in patients with COVID-19. Epidemiol Infect 2022;150:e106. [Crossref] [PubMed]
- Wang S, Ma P, Zhang S, et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia 2020;63:2102-11. [Crossref] [PubMed]
- Liu SP, Zhang Q, Wang W, et al. Hyperglycemia is a strong predictor of poor prognosis in COVID-19. Diabetes Res Clin Pract 2020;167:108338. [Crossref] [PubMed]
- Kohansal Vajari M, Shirin M, Pourbagheri-Sigaroodi A, et al. COVID-19-related coagulopathy: A review of pathophysiology and pharmaceutical management. Cell Biol Int 2021;45:1832-50. [Crossref] [PubMed]
- Jin X, Duan Y, Bao T, et al. The values of coagulation function in COVID-19 patients. PLoS One 2020;15:e0241329. [Crossref] [PubMed]
- Tekle E, Gelaw Y, Dagnew M, et al. Risk stratification and prognostic value of prothrombin time and activated partial thromboplastin time among COVID-19 patients. PLoS One 2022;17:e0272216. [Crossref] [PubMed]
- Kornblith LZ, Sadhanandhan B, Arun S, et al. γ' fibrinogen levels as a biomarker of COVID-19 respiratory disease severity. Blood Cells Mol Dis 2023;101:102746. [Crossref] [PubMed]
- Citu C, Burlea B, Gorun F, et al. Predictive Value of Blood Coagulation Parameters in Poor Outcomes in COVID-19 Patients: A Retrospective Observational Study in Romania. J Clin Med 2022;11:2831. [Crossref] [PubMed]
- Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol 2020;7:e575-82. [Crossref] [PubMed]
- Yu HH, Qin C, Chen M, et al. D-dimer level is associated with the severity of COVID-19. Thromb Res 2020;195:219-25. [Crossref] [PubMed]
- Poudel A, Poudel Y, Adhikari A, et al. D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19. PLoS One 2021;16:e0256744. [Crossref] [PubMed]
- Gao L, Jiang D, Wen XS, et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res 2020;21:83. [Crossref] [PubMed]
- Kaufmann CC, Ahmed A, Kassem M, et al. Mid-regional pro-atrial natriuretic peptide independently predicts short-term mortality in COVID-19. Eur J Clin Invest 2021;51:e13531. [Crossref] [PubMed]
- Malik P, Patel U, Patel NH, et al. Elevated cardiac troponin I as a predictor of outcomes in COVID-19 hospitalizations: a meta-analysis. Infez Med 2020;28:500-6. [PubMed]
- Park M, Hur M, Kim H, et al. Soluble ST2 as a Useful Biomarker for Predicting Clinical Outcomes in Hospitalized COVID-19 Patients. Diagnostics (Basel) 2023;13:259. [Crossref] [PubMed]
- Friedman SA, Charmchi Z, Silver M, et al. Skeletal Muscle Manifestations and Creatine Kinase in COVID-19. Neurohospitalist 2022;12:597-606. [Crossref] [PubMed]
- Zhu F, Li W, Lin Q, et al. Myoglobin and troponin as prognostic factors in patients with COVID-19 pneumonia. Med Clin (Barc) 2021;157:164-71. [Crossref]
- Krishnan A, Prichett L, Tao X, et al. Abnormal liver chemistries as a predictor of COVID-19 severity and clinical outcomes in hospitalized patients. World J Gastroenterol 2022;28:570-87. [Crossref] [PubMed]
- Russo A, Pisaturo M, Monari C, et al. Prognostic Value of Creatinine Levels at Admission on Disease Progression and Mortality in Patients with COVID-19-An Observational Retrospective Study. Pathogens 2023;12:973. [Crossref] [PubMed]
- Liu YM, Xie J, Chen MM, et al. Kidney Function Indicators Predict Adverse Outcomes of COVID-19. Med 2021;2:38-48.e2. [Crossref] [PubMed]
- Singh S, Singh K. Blood Urea Nitrogen/Albumin Ratio and Mortality Risk in Patients with COVID-19. Indian J Crit Care Med 2022;26:626-31. [Crossref] [PubMed]
- Lin L, Chen X, Chen J, et al. The predictive value of serum level of cystatin C for COVID-19 severity. Sci Rep 2021;11:21964. [Crossref] [PubMed]
- Pramana Witarto A, Samarta Witarto B, Er Putra AJ, et al. Serum Krebs von den Lungen-6 for Predicting the Severity of COVID-19 Lung Injury: A Systematic Review and Meta-Analysis. Iran Biomed J 2021;25:381-9. [Crossref] [PubMed]
- d'Alessandro M, Bergantini L, Cavallaro D, et al. Krebs von den Lungen-6 as Disease Severity Marker for COVID-19 Patients: Analytical Verification and Quality Assessment of the Tosoh AIA-360 Compared to Lumipulse G600II. Int J Environ Res Public Health 2022;19:2176. [Crossref] [PubMed]
Cite this article as: Skakun O, Vandzhura Y, Vandzhura I, Symchych K, Symchych A. Biomarkers for unfavourable outcomes prediction in COVID-19 patients: a narrative review. J Emerg Crit Care Med 2024;8:23.


