
Adult croup caused by influenza A virus: a case report and literature review
Highlight box
Key findings
• Adult croup (AC) is a rare condition with a notable female predominance, exhibiting symptoms resembling those of pediatric croup. However, AC is characterized by greater severity compared to its pediatric counterpart, often requiring hospitalization with continuous monitoring due to significant morbidity.
What is known and what is new?
• AC is an extremely rare condition, with limited information available in the literature. Despite being well-documented in pediatric patients, AC is uncommon, with only around 20 cases described to date.
• This case report describes the fourth documented case of AC caused by the influenza A virus. Alongside presenting this novel case, the report provides a thorough literature review covering all previously reported cases of AC.
What is the implication, and what should change now?
• In adult patients with croup, especially those exhibiting symptoms such as fever, barking cough, and stridor, prompt diagnosis and management are crucial, as AC can lead to severe outcomes.
Introduction
Croup is considered a spectrum of upper respiratory tract conditions affecting the larynx, trachea, and bronchi. However, the most representative form of the disease and considered as a distinct clinical entity is laryngotracheitis (1,2).
Influenza is an acute respiratory tract disease caused by influenza type A and B viruses, which have the potential to cause mild to severe illness to epidemics during the winter (3).
Herein, we report a rare case of influenza A virus (IAV) as the causative agent of croup in an adult patient. To the best of our knowledge, only three similar cases have been reported in the literature (4-6).
This article aims to highlight the importance of IAV as a possible etiologic agent of croup in adults. As well as to establish the clear differences that exist between croup in adult and pediatric patients. We present this case in accordance with the CARE reporting checklist (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-62/rc).
Case presentation
A 35-year-old man presented to the emergency department with agitation due to fever, “barking” cough and progressive dyspnea during the last 12 hours. Medical history was significant in requiring medical evaluation 72 hours earlier secondary to being involved in an uncomplicated motor vehicle crash. He reported not having received the seasonal influenza vaccine since 2020.
On admission his vital signs were blood pressure 110/70 mmHg, heart rate 112 beats/min with regular rhythm, respiratory rate 26 breaths/min, oxygen saturation 95% on room air with no evidence of cyanosis and temperature 37.8 ℃. Physical examination revealed use of accessory respiratory musculature and auscultation of biphasic stridor at rest. A chest radiograph was requested, which revealed narrowing of the upper trachea, a finding compatible with the so-called steeple sign (Figure 1). Laboratory tests were significant for a white blood cell count of 14.40 (reference range, 4–10) ×103/mm3. A nasopharyngeal swab was sent for a rapid molecular test which was positive for IAV. A diagnosis of croup due to IAV was made.
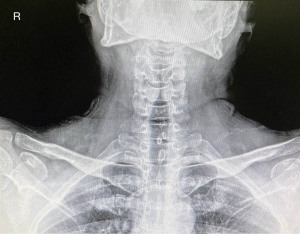
Treatment was started with dexamethasone eight mg intravenous in a single dose since that was the presentation available in our center and there is no standard dose or specific guidelines for the treatment of adult croup (AC), nebulized racemic epinephrine and humidified oxygen. In addition, treatment for influenza A was started with oseltamivir 75 mg orally twice a day for a course of 5 days. The patient was admitted to the intensive care unit (ICU) for close monitoring. The patient showed clinical improvement with resolution of stridor within minutes of administration of racemic epinephrine and recovery of work of breathing during the subsequent three hours.
A control chest X-ray was performed at 24 hours, which showed resolution of the subglottic narrowing, so the patient was transferred from the ICU to regular nursing floor. The patient was discharged on the third day with complete resolution of symptoms and a follow-up visit was scheduled the following week, at which time he remained asymptomatic.
All procedures performed in this study were in accordance with the ethical standards of the Research Ethics Committee of the North Unit Medical School, Autonomous University of Coahuila and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying image. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Croup is frequently observed in pediatric patients, with a predominant incidence in children aged 6 months to 3 years, being unusual outside this age range. Current evidence has reported a male: female ratio in the prevalence of pediatric croup ranging from 1.5:1 to 2:1 (2,7). On the other hand, AC is extremely rare and information describing its characteristics is scarce. To the best of our knowledge, there are only 20 cases described in the literature, of which 12 (60%) were female, 1 (5%) male and 7 (35%) did not provide information (4-6,8-10). Similarly, Tachibana et al. described the clinical characteristics of 18 patients, of whom 15 (83.3%) were female and 3 (16.7%) were male, with a mean age of 43.1 years. One theory proposed to try to explain this large difference in incidence is that even mild subglottic edema would easily cause symptoms in women, given the smaller diameter of the trachea in women compared to men (11).
Approximately 80% of the detectable causes of pediatric croup cases are viral (7). Cases of croup occur more frequently during the fall to winter season, which coincides with a well-recognized increased viral activity causing respiratory illness. Parainfluenza virus (PIV) is the most frequent causative agent of acute laryngotracheitis with a prevalence of approximately 75%, being the most common type one PIV, followed by type two characterized by a milder disease compared to the previous one, and finally type three associated with greater severity in the clinical course. Other viruses involved are rhinovirus, respiratory syncytial virus (RSV) and adenovirus. In recent years, severe acute respiratory syndrome due to coronavirus two, especially the Omicron variant, has stood out for its potential to generate more severe symptoms, to require more intense treatment and more hospital admissions for its management. Nevertheless, croup can rarely be caused by bacteria such as Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae and Corynebacterium diphtheriae (1,7). Patel et al. described the organisms identified in some of the AC patients, with type three PIV, Haemophilus influenzae, influenza virus, Streptococcus and RSV being the most frequent (8). This demonstrates another difference between croup in adult and pediatric patients. Moreover, influenza virus, although considered an infrequent cause of croup, has been associated with longer hospital stays and an increased risk of readmission for recurrence of laryngeal symptoms.
IAV is divided into subtypes according to their two surface proteins, hemagglutinin (HA or H) and neuraminidase (NA or N), with 18 and 11 different subtypes, respectively. It is through this hemagglutinin glycoprotein that the IAV binds to receptors on the surface of respiratory epithelial cells and initiates infection. More than 130 combinations of influenza A subtypes have been identified in nature, however, the most common IAV subtypes in humans are A(H1N1) and A(H3N2) (3,12).
IAV is spread primarily through respiratory contact between individuals within six feet. Transmission via large droplets and smaller aerosols occurs through coughing, sneezing, or talking and can infect others by inhalation or mucosal contact. Risk factors for complications of influenza have been identified, including lack of previous exposure to the viruses, advanced age, long-term care facility residency, children under 5 years of age, pregnancy, obesity, chronic diseases, particularly those of pulmonary or cardiac origin, and immunosuppressive states such as diabetes, human immunodeficiency virus infection and cancer (3).
The pathogenesis of croup begins with the infection of the virus to the epithelium of the nasal mucosa and pharynx, spreading to the larynx, causing inflammation, edema, congestion, and subglottic narrowing, which originates its classically described symptomatology (1). In contrast, the development of AC remains unclear. Risk factors such as impaired immune function associated with human immunodeficiency virus infection, immunosuppressive drugs or physiological changes during pregnancy have been proposed (11).
Croup manifests with a classic triad of “barking” cough, inspiratory stridor, and hoarseness because of laryngeal narrowing. The onset of symptoms is usually sudden, with a peak between 24 and 48 hours, and often worsen at night, although the reason for this peculiarity is unknown, circadian fluctuations in endogenous serum cortisol concentrations have been proposed as a possible explanation. These symptoms are frequently preceded by nonspecific upper respiratory tract symptoms during the previous 12–72 hours such as non-productive cough, sore throat, nausea, nasal congestion, and rhinitis. Interestingly, these symptoms are also found in the context of croup (12). Stridor and mild tachypnea progress to severe respiratory distress in parallel with airway narrowing. Croup symptoms usually resolve quickly, with approximately 60% of children experiencing relief of croup within 48 hours. However, some children may continue to exhibit symptoms for up to 1 week (1,7). Nevertheless, in a retrospective study involving 18 patients with AC, cough and sore throat symptoms were initially reported in 83.3% of patients, while hoarseness, dyspnea, fever with swallowing pain, and stridor were observed in 44.4%, 38.9%, 27.8%, and 5.6% of cases, respectively (11). These findings are compatible with those reported by Patel et al. (8).
In our literature review, we found three cases of AC caused by IAV (Table 1). After inclusion of the present case, out of the four patients, 3 (75%) were female and the mean age was 67.75 years. Among the clinical manifestations, the most frequent were fever, barking cough, stridor, and tachypnea, present in all (100%) of the cases, followed by the use of accessory muscles of respiration in 3 (75%) of the cases, coryza and dyspnea in 2 (50%) of the cases and finally, generalized fatigue and cervical lymphadenopathy reported in only 1 (25%) case. It is important to highlight the large difference in the prevalence of stridor between the cases of AC caused by IAV and those analyzed by Tachibana et al., 100% and 5.6% respectively, which could suggest a more severe presentation and a possible clinical indicator of AC caused by IAV. The steeple sign was reported in all (100%) cases.
Table 1
| Author, year | Age, sex | Clinical manifestations | Laboratory findings | Radiographic findings | Treatment | Clinical course, outcomes |
|---|---|---|---|---|---|---|
| Shaikh et al., 2018, (4) | 88, female | Fever, coryza, barking cough and generalized fatigue for 2 days, fever, tachypnea, supraclavicular and substernal retractions, and audible inspiratory stridor | Monocytosis of 1.2×103/mm3 with a total WBC of 9×103/mm3. Positive rapid influenza A detection test | CXR showed subglottic narrowing of the trachea suggestive of the ‘steeple sign’ | Humidified oxygen, nebulized racemic epinephrine, 10 mg IV dexamethasone once, oseltamivir 75 mg b.i.d for a course of 5 days | Stridor and respiratory distress improved within minutes of racemic epinephrine administration over the course of 4 to 6 hours. CXR repeated a day later showed resolution of subglottic narrowing. She was discharged 2 days later with resolution of symptoms and remained asymptomatic 1 week later |
| Hongo et al., 2019, (5) | 50, female | Fever, coryza, barking cough, stridor, neck lymphadenopathy, and tachypnea with the use of accessory muscles of respiration | ABG analysis showed: pH 7.271; PaO2 82.5 mmHg (FiO2: 1.0); PaCO2 57.6 mmHg; HCO3 −25.6 mmol/L; base excess −1.8 mmol/L; lactate level 1.1 mmol/L. Positive for influenza type A | CXR and CT showed subglottic narrowing of the trachea suggestive of steeple sign | Intubation with a 6-mm endotracheal tube and inhaled epinephrine | Clinical improvement following intubation. She was transferred to another hospital with an otolaryngology unit on the same day |
| Kashiura et al., 2022, (6) | 98, female | Dyspnea, barking cough, tachypnea, hypoxemia, and stridor | Positive rapid diagnostic test for influenza A | CXR and neck CT demonstrated subglottic stenosis, indicated by the so-called steeple sign | Intubation with a 6.5-mm tracheal tube and peramivir IV (600 mg/day) for 5 days | She was extubated on the seventh day |
| Canales-Azcona et al., 2024 (present case) | 35, male | Fever, “barking” cough and progressive dyspnea during the last 12 hours, tachypnea, use of accessory respiratory musculature and biphasic stridor at rest | WBC of 14.40 (reference range, 4–10) ×103/mm3. Positive rapid molecular test for influenza A | CXR revealed narrowing of the upper trachea, a finding compatible with the so-called steeple sign | Dexamethasone 8 mg IV single dose, nebulized racemic epinephrine, humidified oxygen, and oseltamivir 75 mg PO b.i.d for a course of 5 days | Clinical improvement after admission to ICU where racemic epinephrine administration resolved stridor and respiratory distress over the next 3 hours. He was discharged from ICU after 24 hours showing resolution of subglottic narrowing in the control CXR. He was later transferred to the ward and subsequently discharged on the third day with complete resolution of symptoms. On a follow-up visit one week later he remained asymptomatic |
WBC, white blood cell; CXR, chest X-ray; IV, intravenous; b.i.d, twice a day; ABG, arterial blood gas; CT, computed tomography; PO, oral administration; ICU, intensive care unit.
Croup diagnosis is eminently clinical. Laboratory tests, rapid antigen tests or viral cultures, as well as radiological studies are not considered necessary to confirm the diagnosis when typical clinical features of croup are present and acute management should not be delayed. Complementary studies should be reserved for cases in which the course is atypical or the response to initial treatment is ineffective. A complete blood count may demonstrate a variable white blood cell count, although numbers >10,000 cells/microL are common. In the differential diagnosis of croup, the presence of lymphocytosis may suggest a viral etiology, while the presence of banded neutrophils may point to a bacterial infection. A subglottic narrowing commonly referred to as the “steeple sign” demonstrated on a posterior-anterior chest X-ray is often associated with croup in children (1,7). Atypical lymphocytosis usually observed in severe acute viral infections was only reported in 22% of the cases described by Tachibana et al. (11). According to Patel et al., the radiographic finding called “steeple sign” was more frequent in adults, occurring in 92% of the cases analyzed (8). Direct laryngoscopy may be helpful when considering differential diagnoses such as epiglottitis or in severe cases requiring advanced airway management (8,11). However, we agree with Shaikh et al. that this procedure can be avoided in cases highly suggestive of croup in which initial management is initiated immediately (4).
The diagnosis of influenza is based on clinical signs and local epidemiological data, yet laboratory testing is often necessary due to non-specific symptoms. It is particularly important that specimens be obtained for testing in high-risk patients, including patients with or without fever, exacerbation of underlying chronic illness, history of recent travel from areas with known influenza activity, known complications of influenza, or if the test result will influence decisions regarding treatment or hospital stay. Common methods of specimen collection include nasopharyngeal, nasal and throat swabs. Multiple assays are available to establish the diagnosis of influenza; however, Infectious Diseases Society of America (IDSA) guidelines recommend using nucleic acid amplification testing in outpatients and reverse-transcription polymerase chain reaction in hospitalized patients to improve detection of influenza virus infection (13).
AC represents a more severe disease, some authors suggest that it is a criterion for admission to the ICU when available (14,15), however, hospitalization with continuous monitoring has shown good outcomes. Although there is no consensus that provides recommendations for adult cases, treatment of croup, regardless of etiology, is aimed at symptomatic relief by decreasing subglottic inflammation through the administration of glucocorticoids and nebulized epinephrine without delay (4). Dexamethasone is usually the glucocorticoid of choice as it is the most extensively studied agent, with the longest duration of action (24–48 hours) and can be administered orally, intravenously, or intramuscularly, showing effect two to three hours after administration (2,16). Other acceptable options are prednisolone and budesonide (11). The use of nebulized epinephrine is reasonable. According to the literature, both racemic and L-epinephrine have similar efficacy, therefore the use of either is acceptable and it is determined by availability. Interestingly, the therapeutic effects are seen within 1-minute post-administration, although the elimination half-life is 2 hours (2,16). Supplemental oxygen is indicated in patients with hypoxemia (oxygen saturation <92%) or with evidence of respiratory distress. Other therapies include a helium-oxygen mixture known as heliox and humidified air, however there is no evidence to support its routine use (1,8,16).
According to IDSA guidelines, antiviral treatment should be initiated as soon as possible in patients with suspected or confirmed influenza, regardless of history of influenza vaccination, indicated in hospitalized patients regardless of the duration of illness while in outpatients it will be based on the presence of comorbidities, risk of complications from influenza, extreme age or conditions such as pregnancy or postpartum. The preferred antiviral agent is oseltamivir, with a recommended dose of 75 mg orally every 12 hours for 5 days, advised for both adults and children over 12 years of age (13).
Conclusions
AC represents an uncommon clinical entity, especially cases caused by IAV. Our case reinforces the importance of understanding this disease, since it requires early diagnosis and treatment, and unlike croup in pediatric patients, in adults it is a severe disease and has an impact on the patient’s outcome and prognosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-62/rc
Peer Review File: Available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-62/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jeccm.amegroups.com/article/view/10.21037/jeccm-24-62/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Research Ethics Committee of the North Unit Medical School, Autonomous University of Coahuila and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying image. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bjornson CL, Johnson DW. Croup. Lancet 2008;371:329-39. [Crossref] [PubMed]
- Cherry JD. Clinical practice. Croup. N Engl J Med 2008;358:384-91. [Crossref] [PubMed]
- Krammer F, Smith GJD, Fouchier RAM, et al. Influenza. Nat Rev Dis Primers 2018;4:3. [Crossref] [PubMed]
- Shaikh N, Dy P, Basnet A, et al. Uncommon presentation of a common disease: influenza A presenting as adult croup. BMJ Case Rep 2018;2018:bcr2017223974. [Crossref] [PubMed]
- Hongo T, Fujiwara T. Influenza A-induced croup in an adult. J Gen Fam Med 2019;20:213-4. [Crossref] [PubMed]
- Kashiura M, Amagasa S, Moriya T. The Steeple Sign of Croup in an Adult. Intern Med 2022;61:2825. [Crossref] [PubMed]
- Smith DK, McDermott AJ, Sullivan JF. Croup: Diagnosis and Management. Am Fam Physician 2018;97:575-80.
- Patel JJ, Kitchin E, Pfeifer K. A Narrowing Diagnosis: A Rare Cause of Adult Croup and Literature Review. Case Rep Crit Care 2017;2017:9870762. [Crossref] [PubMed]
- Alhedaithy AA, Murad IS, Aldabal N. Acute laryngotracheitis caused by COVID-19: A case report and literature review. Int J Surg Case Rep 2022;94:107074. [Crossref] [PubMed]
- Tanaka Y, Hirano K, Sekino E, et al. Case Report: A 29-Year-Old Pregnant Woman at 24 Weeks of Gestation Presenting with Laryngotracheitis and COVID-19 Due to the R.1 Variant of SARS-CoV-2. Am J Case Rep 2022;23:e937834. [Crossref] [PubMed]
- Tachibana T, Orita Y, Makino T, et al. Prognostic factors and importance of recognition of adult croup. Acta Otolaryngol 2018;138:579-83. [Crossref] [PubMed]
- Javanian M, Barary M, Ghebrehewet S, et al. A brief review of influenza virus infection. J Med Virol 2021;93:4638-46. [Crossref] [PubMed]
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaa. Clin Infect Dis 2019;68:895-902. [Crossref] [PubMed]
- Beckwith SR. A case of adult croup. Intern Emerg Med 2008;3:387-9. [Crossref] [PubMed]
- Woo PC, Young K, Tsang KW, et al. Adult croup: a rare but more severe condition. Respiration 2000;67:684-8. [Crossref] [PubMed]
- Ortiz-Alvarez O. Acute management of croup in the emergency department. Paediatr Child Health 2017;22:166-73. [Crossref] [PubMed]
Cite this article as: Canales-Azcona GA, Ibarra-Sifuentes HR, Ríos-López VV, Castellanos-Maldonado A, Rodríguez-Sifuentes JA, Gómez-Arredondo JI. Adult croup caused by influenza A virus: a case report and literature review. J Emerg Crit Care Med 2024;8:26.


